=================================================================================
The sandwich structure shown in Figure 2321 (a) is composed of three layers: SrTiO3, PbTiO3 (lead titanate), and Pt. An amorphous Ti-rich interfacial layer as well as nanometer size precipitates was formed at PbTiO3/Pt interfaces. In the low-loss region in EELS from the different layers shown in Figure 2321 (b), the energy peaks labeled A–H for SrTiO3 and a–h for PbTiO3 are formed by interband transitions which are typical in bulk SrTiO3 and PbTiO3 [1]. The strong peaks H/h and H’/h’ arise from transitions of Ti 3p to higher energy levels such as Ti 3d, Ti 4s, forming Ti M2,3 edge. In the precipitates, the transitions h and h are reduced in intensity because these precipitates might be Ti-deficient. In Figure 2321 (c), the two core loss EELS profiles from SrTiO3 and PbTiO3 layers show clear splitting of the Ti-L2,3 edges, while for the interfacial layer the splitting at the Ti-L3 edge is much less and the splitting at the Ti-L2 edge disappears. The splitting of Ti-L2,3 edges reflects the hybridization and ligand field strength of Ti–O atomic interaction so that the reduction of splitting in the interfacial layer reflects the weaker Ti–O bonding force. Due to the similar hybridization of O 2p states with Ti 3d in the conduction band, the energy region from 530 eV to 536 eV is split into two subbands t2g (marked peak 1) and eg (marked peak 2). Figure 2321 (d) shows the EEL spectra of Pb- and Pt-M4,5 edges.
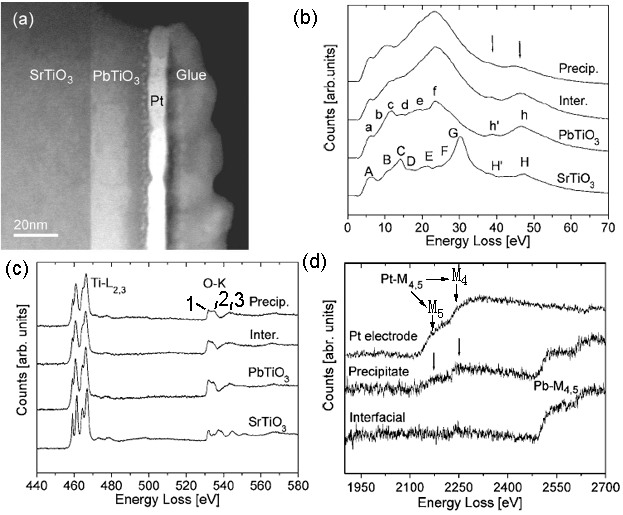
Figure 2321. (a) TEM image of sandwich structure ( SrTiO3, PbTiO3, and Pt), and (b) low-loss region in EELS, (c) Ti-L2,3 and O-K edges, and (d) Pb- and Pt-M4,5 edges
from the different layers. The black arrows in (d) denote the positions of Pt-M4,5 edges. Adapted from [2]
Note that strong ferroelectricity in PbTiO3 is formed due to a mechanism driven by the A site in the perovskite, that is, the 6s2 electrons of Pb2+ hybridize with the O-2p electrons to form strong covalent bonds, resulting in a relative displacement of the Pb2+ cage with respect to the O-octahedron.
[1] K. V. Benthem, C. Elsasser, and R. H. French, J. Appl. Phys. 90, 6156 (2001).
[2]
L. F. Fu, S. J. Welz, and N. D. Browning, M. Kurasawa, and P. C. McIntyre, Z-contrast and electron energy loss spectroscopy study of passive layer formation at ferroelectric PbTiO3/Pt interfaces, Applied Physics Letters, 87, 262904 (2005).
|
