=================================================================================
The K family in X-rays is composed of two recognizable lines Kα and Kβ for energies above 3 keV. Kα X-ray is emitted when vacancy in K-shell is filled by a transition from L-shell, while Kβ X-ray is emitted when vacancy in K-shell is filled by a transition from M-shell. The ratio of the intensities of the Kα peak to Kβ peak is about 10:1. When those peaks are resolved their ratio should be apparent in the identification of an element. Any significant deviation from this ratio should be considered as elemental misidentification or the presence of a second element.
Figure 4683 shows an example of the ionization processes and generations of X-rays. A high-energy electron (incident electron) must penetrate through the outer conduction/valence bands and interact with the inner-shell (or core) electrons. If the high-energy electron transfers more than a critical amount of energy to an inner-shell electron (K electron here), that electron is ejected into the vacuum, that is, it escapes (step 2b in the figure) the attractive field of the nucleus, leaving a hole in the inner shell (K shell in the figure) and escapes above the Fermi level into the unfilled states. In this case, the atom is ionized. The excited atom can return almost to its ground state (lowest energy) by filling in the hole with an electron from an outer shell (step 3). This transition is accompanied by the emission of an X-ray in Figure 4683. The energy of the X-ray emission is characteristic of the difference in energy between the two electron shells involved (L3 → K in the figure) and this energy difference is unique to the specific atom.
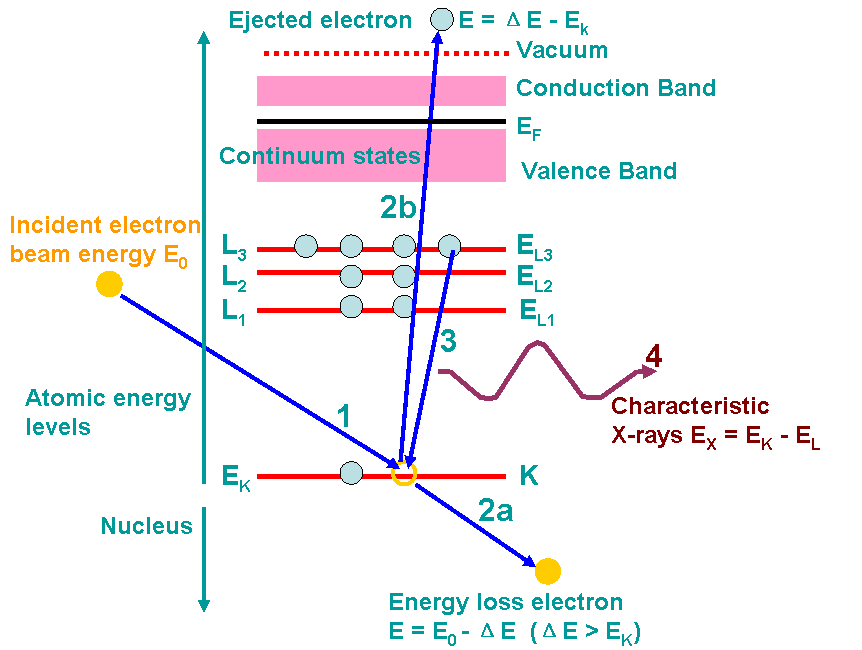
Figure 4683. Example of the ionization processes and generations of X-rays. The numbers indicates the process sequence. In this process, an inner (K) shell electron is ejected
from the atom by a high-energy electron (incident electron). In step 3, the hole in the K shell
is filled by an electron from the L shell (L3 here), characteristic (Kα) X-ray emission
occurs (step 4). The beam electron loses energy but continues on through the
specimen (step 2a). Steps 2a and 2b almost occur at the same time.
If neither a proper database nor standards are available, the kAB factors can be theoretically calculated,
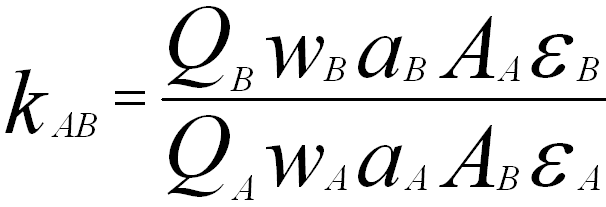 ----------------------------- [4683] ----------------------------- [4683]
where,
QA and QB -- The ionization cross-sections for the X-rays of elements A and B, respectively,
wA and wB -- The fluorescence yields for elements A and B, respectively,
aA and aB -- The
relative transition probabilities for elements A and B, respectively,
AA and AB -- The atomic weights for elements A and B, respectively,
εA and εB -- The detection efficiencies of the
EDS detector for the X-rays from elements A and B, respectively.
In Equation 4683, it is most difficult to accurately calculate Q and ε. The extraction of the kAB values for K lines above 1.5 eV in energy is in error of ~10 to 15% mainly due to the inaccurate estimation of Q. For the same reason, it is not recommended to calculate the kAB values for light elements Z < 11 or for L lines.
|

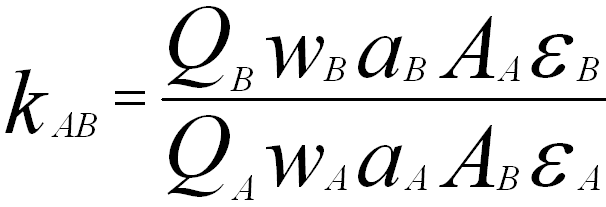 ----------------------------- [4683]
----------------------------- [4683]