=================================================================================
Two types of X-rays are induced by the electron beam: characteristic X-rays and Bremsstrahlung X-rays as shown in Figure 4689a. Characteristic X-rays are very useful and convenient for local elemental analysis of crystal defects, precipitates, and nano-structured materials. A small fraction, about 1 electron in 103 electrons or less, of the electron interactions, ionizes the atoms and may result in emission of a characteristic photon, including X-ray. These X-rays are nominally sharp lines.
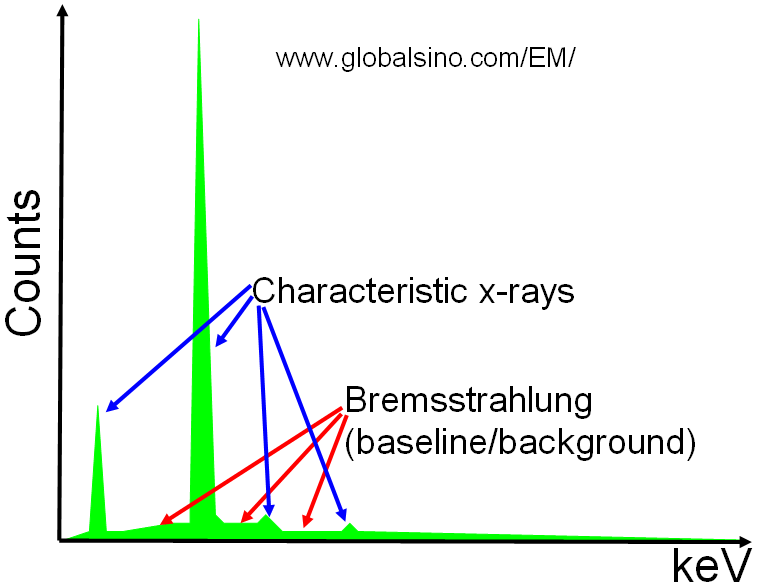
Figure 4689a. Schematic showing X-rays of a sample element.
Figure 4689b shows an example of the ionization processes and generations of X-rays. A high-energy electron (incident electron) must penetrate through the outer conduction/valence bands and interact with the inner-shell (or core) electrons. If the high-energy electron transfers more than a critical amount of energy to an inner-shell electron (K electron here), that electron is ejected into the vacuum, that is, it escapes (step 2b in the figure) the attractive field of the nucleus, leaving a hole in the inner shell (K shell in the figure) and escapes above the Fermi level into the unfilled states. In this case, the atom is ionized. The excited atom can return almost to its ground state (lowest energy) by filling in the hole with an electron from an outer shell (step 3). This transition is accompanied by the emission of an X-ray in Figure 4689b. The energy of the X-ray emission is characteristic of the difference in energy between the two electron shells involved (L3 → K in the figure) and this energy difference is unique to the specific atom.
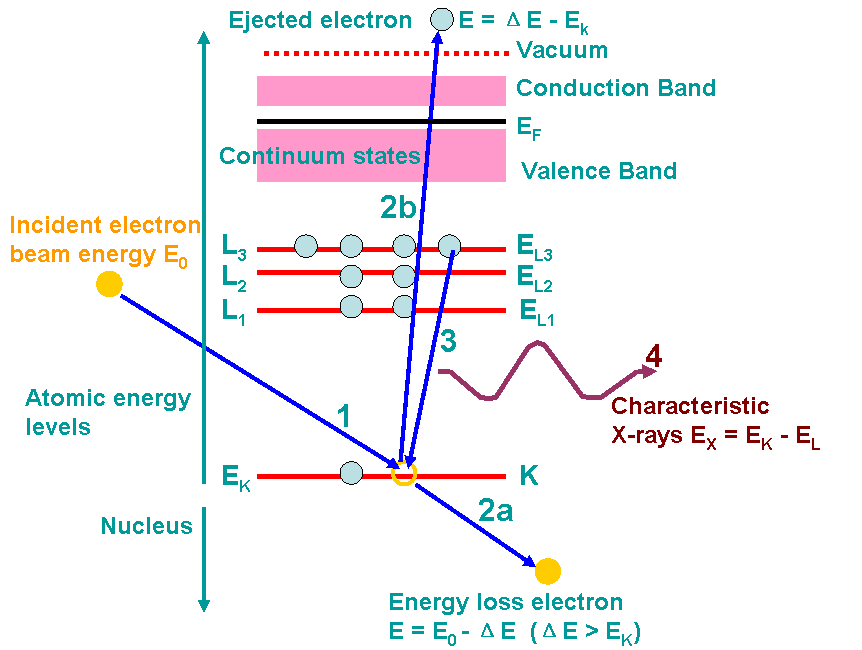
Figure 4689b. Example of the ionization processes and generations of X-rays. The numbers indicates the process sequence. In this process, an inner (K) shell electron is ejected
from the atom by a high-energy electron (incident electron). In step 3, the hole in the K shell
is filled by an electron from the L shell (L3 here), characteristic (Kα) X-ray emission
occurs (step 4). The beam electron loses energy but continues on through the
specimen (step 2a). Steps 2a and 2b almost occur at the same time. ΔE is the energy loss of the incident electron.
The energy, EX, of an X-ray photon in Figure 4689b is given by
EX = hν ---------------------------------------------------------------------- [4689a]
Where, h is the Planck’s constant and the frequency ν=c/λ. Here, c is the speed of light in vacuum (2.99793 x 108 m/sec) and λ the wavelength of the X-ray. Thus, the wavelength is related to the energy as given by,
λ=hc/EX = 12.398/EX ------------------------------------------------------- [4689b]
Where EX is in keV.
The characteristic X-Rays of all the elements are listed at Detailed energy & intensity (weight/yield) of K, L, & M lines.
The peaks in the X-ray spectra, detected by the EDS detector, are described by Gaussian distribution,
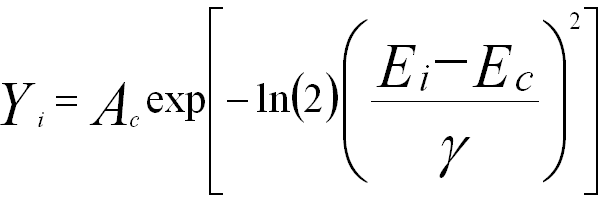 ---------------- [4689c] ---------------- [4689c]
where,
Ec -- The energy at the center of the peak,
Ei -- The energy at the ith channel,
Yi -- The amplitude in the ith channel,
Ac -- The amplitude at the center of the peak,
γ -- The 1/2*FWHM (full width at half maximum).
In general, EDS has poor energy resolution of the peaks. A typical EDS peak is about 100 times the natural peak width, which is limited by the statistics of electron-hole pair production and electronic noise, resulting in severe peak overlaps.
|


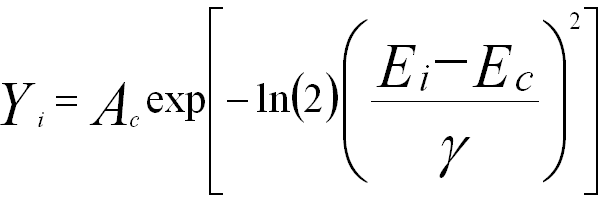 ---------------- [4689c]
---------------- [4689c]