=================================================================================
Inner shell excitations occur at energies:
ΔE ≥ EF - EB ----------------------------------- (4725)
where,
ΔE -- The energy loss
EF -- The Fermi level energy
EB -- Binding energy of the inner shell
The EELS feature corresponds to "core loss edges" (ΔE = EF - EB), see Figure 4725b below.
The EELS edge onset is the sudden rise in intensity preceding peaks, representing the ionization threshold which approximately corresponds to the inner-shell binding energy. Energy loss of penetrating electrons occurs due to inner-shell electron excitation. The excitation of atomic inner shells enables us to study the unoccupied conduction states in a solid. These core-level processes are mostly sensitive to the final states since the initial states have narrow energy widths. In EELS profiles, the ionization edges induced by the inner-shell excitation represents ionization threshold and reflects the inner-shell binding energy as shown in Figure 4725a.
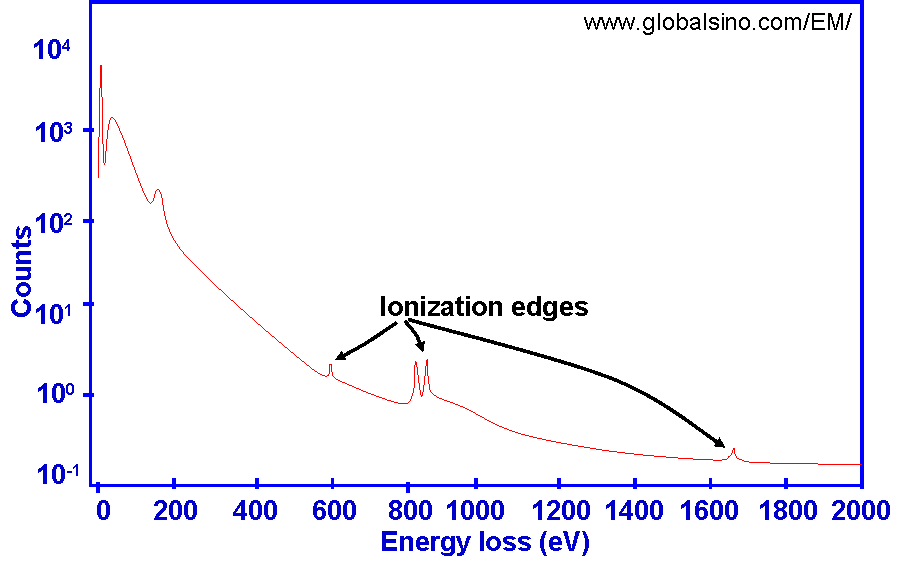
Figure 4725a. Ionization edges induced by inner-shell excitation.
Figure 4725b shows the schematic illustration of an energy-loss spectrum and the formation of the main energy-loss peaks related to the energy levels of electrons surrounding atom A and atom B in materials.
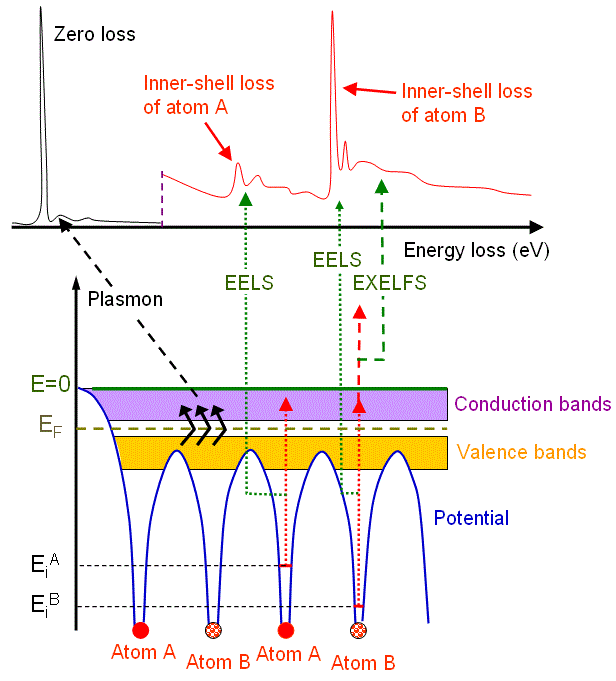
Figure 4725b. Schematic illustration of an energy-loss spectrum and the formation of three main energy-loss peaks.
The ground-state energy of an inner-shell electron is typically some hundreds to thousands of electron volts (eV) below the Fermi level of the solid and the unoccupied electrons states lies only above the Fermi level. When an accelerating electron in the electron beam in EMs interacts with the materials, the inner-shell electrons can be excited and transit to an excited state if the electrons absorb an energy which is equal to or greater than its binding energy. In this case, the accelerating electron in the electron beam loses the same amount of energy and is scattered at an angle. However, these unstable excited electrons will lose its excess energy quickly, producing “byproducts” such as X-rays and Auger electrons. This process is called de-excitation process.
An energy filtering transmission microscope (EFTEM) can provide information about the spatial distribution of an element by selecting an
energy loss corresponding to a characteristic inner-shell excitation. Due to the smaller ionization cross section, the scattering intensity of inner-shell electron excitation is relatively low and thus, the fast incident electrons have a long mean free path compared to the TEM specimen thickness. Therefore, the probability of plural and multiple inner-shell excitations is negligible.
|