=================================================================================
Table 2023a. Properties of silver (Ag).
Resistivity (10-6 Ω•cm) |
1.59 |
|
Corrosion in air |
Poor |
Melting point (°C) |
962 |
|
Specific heat capacity(Jkg-1K-1) |
234 |
Thermal conductivity (Wcm-1) |
4.25 |
|
Adhesion to SiO2 |
Poor |
Thermal stress per degree for
films on silicon (107 dyn cm-2 °C-1) |
1.9 |
|
Youngs modulus
(10-11 dyn cm-2) |
8.27 |
Work Function (eV) |
4.26 |
|
Electronegativity |
1.93 |
Coefficient of thermal expansion (CTE)
(10-6 °C-1) |
19.1 |
|
Density (g-cm-3) |
10.5 |
Heat conductivity (kh,Wcm-1K-1) |
4.29 |
|
Vaporization temperature (Tv, °C) |
2212 |
Melt heat (Qm, J-g-1) |
105 |
|
Vaporization heat (Qv, J-g-1) |
2387 |
Absorption (1-R) |
0.03 |
|
Kα X-ray for XRD measurements |
Wavelength (λ) = 0.056 nm |
Table 2023b. Reflection angles (°) for silver (Ag, a = 4.08650(2) Å) (λ = 1.5405929 Å and T = 298 K).
| hkl |
Reflection angles (°) |
| 111 |
38.112 |
| 200 |
44.295 |
| 220 |
64.437 |
| 311 |
77.390 |
| 222 |
81.533 |
| 400 |
97.875 |
| 331 |
110.499 |
| 420 |
114.914 |
| 422 |
134.871 |
| 511/333 |
156.737 |
In general, diffusion coefficient is greater through less restrictive structural regions [1]. For instance, the diffusion coefficient through surface is greater than that through grain boundary, while it is greater through grain boundary than through single crystal as indicated in Figure 2023.
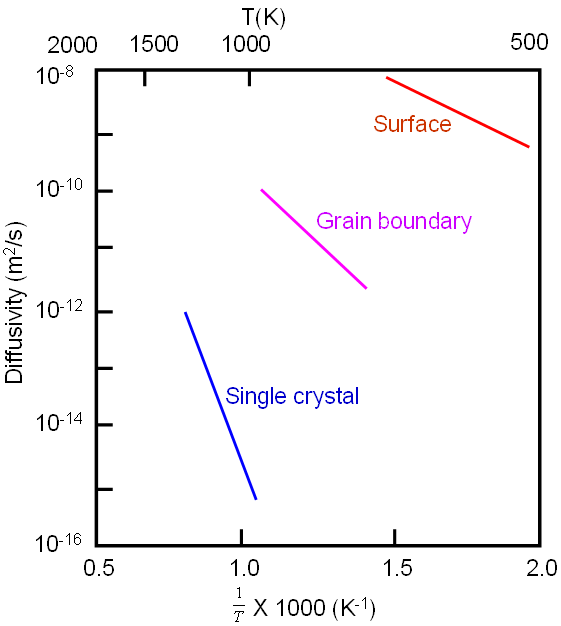
Figure 2023. Self-diffusion coefficients for silver (Ag) depend on the diffusion path. Adapted from [1]
[1] J. H. Brophy, R. M. Rose, and J. Wulff, The Structure and Properties of Materials, Vol. 2: Thermodynamics of Structure, John Wiley & Sons, Inc., New York, 1964.
|