=================================================================================
The name “perovskite” originally refers to a type of minerals with chemical formula CaTiO3, named after Russian mineralogist L. A. Perovski. In general, any crystals with the same structure as CaTiO3 are categorized into the perovskite structure.
Most perovskite crystals are oxides with the general formula ABO3, where A and B represent two different cations and O represents oxygen elements. The A-site cations are larger than B-site cations. Perovskite oxides (ABO3) can be described as the framework of corner-shared BO6 octahedra with 12-coordinated A cations. The unit cell of an ideal perovskite structure is a cube, where the A-site cations locate at the corners of the cube, the B-site cation sits in the body center and oxygen sits in the face centers as shown in Figure 3533a. The structure is a corner-linked oxygen octahedral network. The octahedra are linked in a regular cubic array, resulting in high-symmetry cubic m-3m prototype structure. The 6-fold-coordinated B-site in the center of the oxygen octahedron is occupied by a small highly charged cation with a valence state of 3+, 4+, 5+ or 6+, and the larger 12-fold coordinated A-site between octahedra is occupied by a larger cation with a valence state of 1+, 2+, or 3+.
The main advantage of the perovskite structures is the large flexibility in tailoring the chemical composition and lattice parameter(s) of the system by substituting the different cations that present on both the A and B sites without changing the overall structure completely.
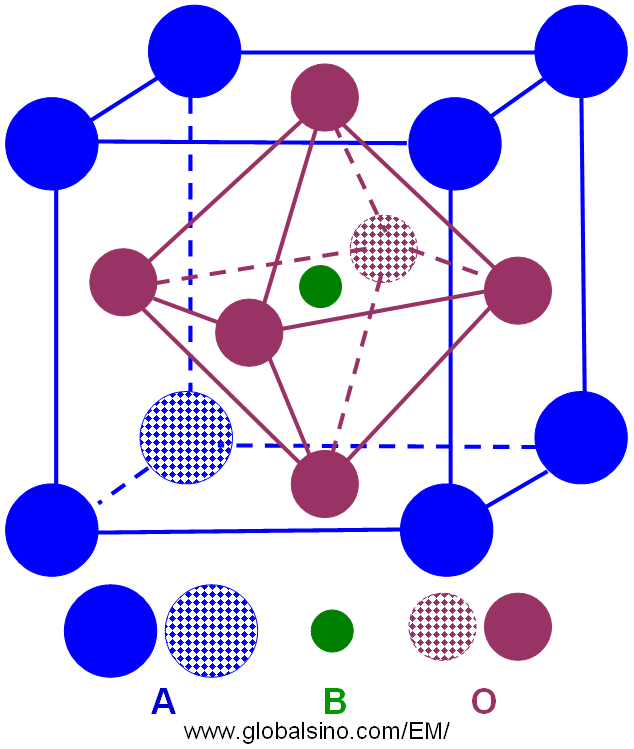
Figure 3533a. Schematic illustration of ideal perovskite structure.
However, most perovskite structures do not have such ideal cubic symmetry and are normally distorted, even for the prototype perovskite, CaTiO3. For instance, the properties of the cations on the A-sites and on the B-sites often induce common distortions such as cation displacements within the octahedra and tilting of the octahedra. In general, the degree of distortion in ABO3 perovskites can be determined by [1],
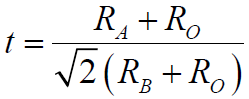 ----------------------------- [3533] ----------------------------- [3533]
where,
RA, RB and RO -- The ionic radii of the A-site cation, B-site cation and oxygen anion, respectively.
t -- Goldschmidt tolerance factor.
In an ideal cubic perovskite crystalline structure the A-site and B-site cations optimize their equilibrium bond distances to the oxygen elements without inducing any distortion of the unit cell at t = 1. When 0.75 < t < 1.05 (that almost all the perovskites have), a distorted perovskite structure can normally be stabilized. Note that most of the cubic perovskites have t values in between 0.8 and 0.9.
All the ferroelectric materials today are based on corner-linked oxygen octahedral structures. The simplest configuration is the well-known perovskite structure. In EELS, perovskite type ferroelectric and high-k dielectric materials, such as BaTiO3 and SrTiO3, normally show only one interband plasmon peak [2–5].
For perovskite cell structures, octahedral oxygen (O) bonding around the atom (Mn for LaMnO3 as shown in Figure 3533b) in B-site generates the crystal field around the B-site atom. The valence states of the atoms (e.g. Mn) are fixed by charge neutrality. In the case of LaMnO3, if the parent compound is doped with Sr2+ for La3+, then Mn3+ (d3) is replaced by Mn4+ in the lattice.
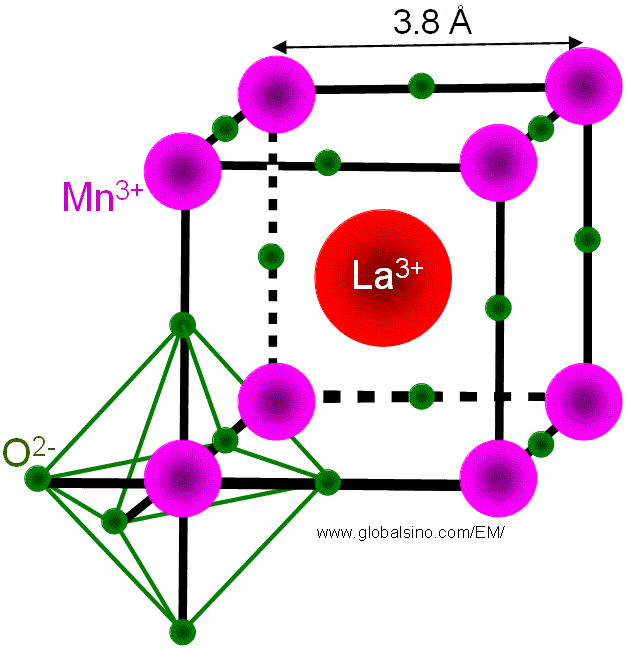
Figure 3533b. Octahedral oxygen bonding around the Mn atom in LaMnO3.
Table 3533a. Atomistic positions in cubic perovskites.
| Site |
Location |
Co-ordinates |
| A cation |
(2a) |
(0, 0, 0) |
| B cation |
(2a) |
(1/2 , 1/2 , 1/2) |
| O anion |
(6b) |
(1/2 , 1/2 , 0) (1/2 , 0, 1/2) (0, 1/2 , 1/2 ) |
Table 3533b. Atomistic positions in orthorhombic perovskites.
| Site |
Location |
Co-ordinates |
| A cation |
(4c) |
±[(u, v, 1/4)(1/2-u, v+1/2, 1/4)] |
| B cation |
(4b) |
(1/2, 0, 0) (1/2, 1/2, 0) (0, 0, 1/2) (0, 1/2, 1/2) |
| O(1) anion |
(4c) |
±[(m, n, 1/4) (1/2-m, n+1/2, 1/4)] |
| O(2) anion |
(8d) |
±
[(x, y, z) (1/2-x, y+1/2, 1/2-z) (-x, -y, z+1/2) (x+1/2, 1/2-y, -z)] |
| * u, v, m, n are dependent on the particular structure under consideration. |
Table 3533c. Atomic positions for rhombohedral perovskites.
| Site |
Location |
Co-ordinates |
| A cation |
(6a) |
(0, 0, 1/4) |
| B cation |
(6b) |
(0, 0, 0) |
| O anion |
(18e) |
(x, 0, 1/4) |
| * The co-ordinates are based on hexagonal axes. |
Table 3533d. Atomic positions for hexagonal perovskites.
| Site |
Location |
Co-ordinates |
| A cation |
2a |
(0, 0, z) |
| A cation |
4b |
(1/3 , 2/3 , z) |
| B cation |
6c |
(x, 0, z) |
| O(1) anion |
6c |
(x, 0, z) |
| O(2) anion |
6c |
(x, 0, z) |
| O(3) anion |
2a |
(0, 0, z) |
| O(4) anion |
4b |
(1/3 , 2/3 , z) |
Table 3533e. Examples of perovskite crystals.
Substance |
A-site |
B-site |
Mobile ions |
Space Group |
Crystal structure |
Lattice constant (nm) |
Angles |
Ionic conductivity/Scm-1 (at °C) |
Remarks |
| Ag3SI |
|
|
Ag+ |
|
|
|
|
1x10-2 at 25 |
No-oxide anti-perovskite-type |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF) |
Ba & Sr |
Co & Fe |
|
|
|
|
|
|
Good catalytic activity above 600 °C |
| BaTiO3 |
|
|
|
|
|
0.401 |
|
|
|
| BiFeO3 |
|
|
|
R3CH |
Rhombohedral |
a = 0.55775; b = 0.55775; c = 1.38616 |
90, 90, 120 |
|
|
| CaCu3Ti4O12 |
Ca2+ & Cu2+ |
|
|
|
|
|
|
|
High permittivity: good for capacitors |
| CaSnO3 |
|
|
|
|
|
0.392 |
|
|
|
| CaTiO3 |
|
|
|
|
|
0.384 |
|
|
|
| CaZrO3 |
|
|
|
|
|
0.402 |
|
|
|
| CeAlO3 |
|
|
|
P4/mmm |
|
a = 0.37669; c = 0.37967 |
|
|
|
| CeCrO3 |
|
|
|
Pm-3m |
|
0.389 |
|
|
|
| CeFeO3 |
|
|
|
|
Rhombohedral |
0.39 |
90, 90, 90 |
|
|
| CeGaO3 |
|
|
|
|
|
0.3879 |
|
|
|
| CeVO3 |
|
|
|
Pbnm |
|
a = 0.5514; b = 0.5557; c = 0.7808 |
|
|
|
| CrBiO3 |
|
|
|
|
Tetragonal |
a = 0.777; c = 0.808 |
90, 90, 90 |
|
|
| CsCdBr3 |
|
|
|
|
|
0.533 |
|
|
|
| CsHgBr3 |
|
|
|
|
|
0.577 |
|
|
|
| CsHgCl3 |
|
|
|
|
|
0.544 |
|
|
|
| CsIO3 |
|
|
|
|
|
0.466 |
|
|
|
| CsPbCl3 |
|
|
Cl- |
|
|
|
|
1.2x10-3 at 500 |
No-oxide perovskite |
| CsPbBr3 |
|
|
Br- |
|
|
|
|
8x10-4 at 500 |
No-oxide perovskite |
| DyAlO3 |
|
|
|
Pbnm |
|
a = 0.521; b = 0.531; c=0.74 |
|
|
|
| DyFeO3 |
|
|
|
Pbnm |
|
a = 0.5302; b = 0.5598; c = 0.7623 |
|
|
|
| DyMnO3 |
|
|
|
Pbnm |
|
a = 0.5842; b = 0.7378; c = 0.528 |
|
|
|
| ErFeO3 |
|
|
|
Pbnm |
|
a = 0.5263; b = 0.5582; c = 0.7591 |
|
|
|
| EuAlO3 |
|
|
|
Pbnm |
|
a = 0.5271; b = 0.5292; c = 0.7458 |
|
|
|
| EuFeO3 |
|
|
|
Pbnm |
|
a = 0.5372; b = 0.5606; c = 0.7685 |
|
|
|
| GdAlO3 |
|
|
|
|
Rhombohedral |
a = 1.056; b = 1.056; c = 1.289 |
90, 90.6, 90 |
|
|
| GdAlO3 |
|
|
|
Pbnm |
|
a = 0.5247; b = 0.5304; c = 0.7447 |
|
|
|
| GdCoO3 |
|
|
|
|
|
a = 0.3732; b = 0.3807; c = 0.3676 |
|
|
|
| GdCrO3 |
|
|
|
Pbnm |
|
a = 0.5312; b 0.5515; c = 0.76 15 |
|
|
|
| GdFeO3 |
|
|
|
Pbnm |
|
a = 0.5349; b = 0.5611; c = 0.7669 |
|
|
|
| GdMnO3 |
|
|
|
|
|
0.382 |
|
|
|
| GdScO3 |
|
|
|
Pbnm |
|
a = 0.5742; b = 0.7926; c = 0.5482 |
|
|
|
| HoFeO3 |
|
|
|
Pbnm |
|
a = 0.5278; b = 0.5591; c = 0.7602 |
|
|
|
| KIO3 |
|
|
|
|
|
0.441 |
|
|
|
| KMgF3 |
|
|
|
|
|
0.397 |
|
|
|
| KNiF3 |
|
|
|
|
|
0.401 |
|
|
|
| KZnF3 |
|
|
|
|
|
0.405 |
|
|
|
| LaAlO3 |
|
|
|
|
|
0.378 |
|
|
|
| LaAlO3 |
|
|
|
R-3c |
Rhombohedral |
a = 0.5.3647; c = 1.31114 |
60.1, 90, 90 |
|
|
| LaCoO3 |
|
|
|
R-3CR |
Rhombohedral |
a = 0.53416; b = 0.53416; c = 0.53416 |
60.99, 60.99, 60.99 |
|
|
| LaCrO3 |
|
|
|
Pbnm |
|
a = 0.5479; b = 0.77562; c = 0.55161 |
|
|
|
| LaFeO3 |
|
|
|
Pbnm |
|
a = 0.55647; b = 0.78551; c = 0.5556 |
|
|
|
| LaGaO3 |
|
|
|
|
|
0.388 |
|
|
|
| LaGaO3 |
|
|
|
Pbnm |
|
a = 0.55245; b = 0.54922; c = 0.7774 |
|
|
|
La0.51Li0.34TiO2.94
|
La & Li |
Ti |
Li+ |
|
|
|
|
1.4x10-3 at 27 |
A-site deficient |
| LaMnO3 |
|
|
|
Pbnm |
|
a = 0.55367; b = 0.57473; c = 0.76929 |
|
|
|
| LaNiO3 |
|
|
|
R-3CH |
Rhombohedral |
a = 0.54573; b = 0.54573; c = 1.31601 |
90, 90, 120 |
|
|
| LaRhO3 |
|
|
|
Pbnm |
|
a = 0.5524; b = 0.5679; c = 0.79 |
|
|
|
La0.9Sr0.1Ga0.8Mg0.2O2.85 |
La & Sr |
Ga & Mg |
O2- |
|
|
|
|
1.5x10-1 at 800 |
Doped single perovskite oxide |
| LaTiO3 |
|
|
|
Pbnm |
|
a = 0.56301; b = 0.55844; c = 0.7901 |
|
|
|
| LaVO3 |
|
|
|
Pbnm |
|
a = 0.55518; b = 0.7848; c = 0.5554 |
|
|
|
| LuFeO3 |
|
|
|
Pbnm |
|
a = 0.5213; b = 0.5547; c = 0.7565 |
|
|
|
| NdAlO3 |
|
|
|
R-3c |
|
a = 0.53223; b = 0.53223; c = 1.29292 |
90, 90, 120 |
|
|
| NdCoO3 |
|
|
|
Pbnm |
|
a = 0.53312; b = 0.75482; c = 0.53461 |
|
|
|
| NdCrO3 |
|
|
|
Pbnm |
|
a = 0.54798; b = 0.76918; c = 0.54221 |
|
|
|
| NdFeO3 |
|
|
|
Pbnm |
|
a = 0.5587; b = 0.7761; c = 0.54505 |
|
|
|
| NdGaO3 |
|
|
|
Pbnm |
|
a = 0.54276; b = 0.54979; c = 0.77078 |
|
|
|
| NdMnO3 |
|
|
|
Pbnm |
|
a = 0.57119; b = 0.7589; c = 0.54119 |
|
|
|
| NdScO3 |
|
|
|
Pbnm |
|
a = 0.5555; b = 0.5744; c = 0.7972 |
|
|
|
| NdVO3 |
|
|
|
Pbnm |
|
a = 0.5461; b = 0.558; c = 0.7762 |
|
|
|
| PrAlO3 |
|
|
|
R-3c |
Rhombohedral |
a = 0.53337; b = 0.53337; c = 1.29766 |
90, 90, 120 |
|
|
| PrCoO3 |
|
|
|
Pm-3m |
|
0.378 |
|
|
|
| PrCrO3 |
|
|
|
Pbnm |
|
a = 0.5444; b = 0.5484; c = 0.771 |
|
|
|
| PrFeO3 |
|
|
|
Pbnm |
|
a = 0.5482; b = 0.5578; c = 0.7786 |
|
|
|
| PrGaO3 |
|
|
|
Pbnm |
|
a = 0.54526; b 0.54947; c = 0.7121 |
|
|
|
| PrMnO3 |
|
|
|
Pbnm |
|
a = 0.5450; b = 0.5786; c = 0.7589 |
|
|
|
| PrMnO3 |
|
|
|
|
|
0.382 |
|
|
|
| PrVO3 |
|
|
|
|
|
a = 0.548; b = 0.559; c = 0.776 |
|
|
|
| PuAlO3 |
|
|
|
|
Rhombohedral |
0.533 |
56.07, 90, 90 |
|
|
| PuCrO3 |
|
|
|
|
|
a = 0.546; b = 0.551; c = 0.776 |
|
|
|
| PuMnO3 |
|
|
|
Pbnm |
|
a = 0.54; b = 0.5786; c = 0.7589 |
|
|
|
| PuVO3 |
|
|
|
Pbnm |
|
a = 0.548; b = 0.561; c = 0.778 |
|
|
|
| RbIO3 |
|
|
|
|
|
0.452 |
|
|
|
| SmCoO3 |
|
|
|
Pm-3m |
|
0.375 |
|
|
|
| ScAlO3 |
|
|
|
Pbnm |
|
a = 0.4937; b = 0.52321; c = 0.72045 |
|
|
|
| SmAlO3 |
|
|
|
Pbnm |
|
a = 0.52912; b = 0.52904; c = 0.7474 |
|
|
|
| SmCrO3 |
|
|
|
Pm-3m |
|
0.386 |
|
|
|
| SmFeO3 |
|
|
|
Pbnm |
|
a = 0.54; b = 0.5597; c = 0.7711 |
|
|
|
| SmVO3 |
|
|
|
|
|
0.389 |
|
|
|
| SmVO3 |
|
|
|
|
|
a = 0.54; b = 0.5591; c = 0.768 |
|
|
|
| SrCe0.95Yb0.05O3-α |
Sr |
Ce & Yb |
H+ |
|
|
|
|
1x10-2 at 900 |
Doped single perovskite oxide + hydrogen |
| SrCoO3-δ |
Sr |
Co |
|
|
|
|
|
|
Used as MIEC cathodes in IT-SOFC |
| SrCo0.8Fe0.2O3-δ (SCF) |
Sr |
Co & Fe |
|
|
|
|
|
|
Good catalytic activity above 600 °C |
| SrCo1−xNbxO3−δ |
Sr |
Co & Nb5+ |
|
|
|
|
|
|
|
| SrTiO3 |
|
|
|
|
|
0.391 |
|
|
|
| SrZrO3 |
|
|
|
|
|
0.410 |
|
|
|
| TbFeO3 |
|
|
|
Pbnm |
|
a = 0.53268; b = 0.55978; c = 0.76406 |
|
|
|
| YAlO3 |
|
|
|
|
|
0.368 |
|
|
|
| YAlO3 |
|
|
|
Pbnm |
|
a = 0.51377; b = 0.52736; c = 0.73085 |
|
|
|
| YbFeO3 |
|
|
|
Pbnm |
|
a = 0.5233; b = 0.5557; c = 0.757 |
|
|
|
| YCrO3 |
|
|
|
Pbnm |
|
a = 0.5247; b = 0.5518; c = 0.754 |
|
|
|
| YFeO3 |
|
|
|
Pbnm |
|
a = 0.52819; b = 0.55957; c = 0.76046 |
|
|
|
| YScO3 |
|
|
|
Pbnm |
|
a = 0.5431; b 0.5712; c = 0.7894 |
|
|
|
In Table 3533a, some dopants are introduced into the perovskite structures for different purposes. For instance, Sb, Mo or Sn are introduced [15-17] at the Co positions in SrCoO3-δ in order to avoid the formation of the unwanted 2H-hexagonal structure. [6-8]
Table 3533f. Other characteristics of perovskite structures.
[1] V. M. Goldschmidt, T. Barth, G. Lunde, and W. Zachariasen, Skrifter Norske Videnskaps-Akad. Oslo, Mat.-Nat. Kl. 2, 117 (1926).
[2] K.S. Katti, M. Qian, F. Dogan, M. Sarikaya, J. Am. Ceram. Soc. 85 (2002)
2236–2243.
[3] K. van Benthem, C. Elsasser, R.H. French, J. Appl. Phys. 90 (2001) 6156–6164.
[4] S. Schamm, G. Zanchi, Ultramicroscopy 88 (2001) 211–217.
[5] J. Zhang, A. Visinoiu, F. Heyroth, F. Syrowatka, M. Alexe, D. Hesse, H.S. Leipner,
Phys. Rev. B 71 (2005) 064108.
[6] Aguadero, A.; Pérez-Coll, D.; Alonso, J.A.; Skinner, S.J.; Kilner, J. A new family
of Mo-doped SrCoO3−δ perovskites for application in reversible solid state
electrochemical cells. Chem Mater 2012, 24, 2655-2663.
[7] Wang, S.F.; Hsu, Y.F.; Yeh, C.T.; Huang, C.C.; Lu, H.C. Characteristics of
SrCo1−xSnxO3−δ cathode materials for use in solid oxide fuel cells. Solid State Ionics
2012, 227, 10-16.
[8] Aguadero, A.; Alonso, J.A.; Perez-Coll, D.; De la Calle, C.; Fernández-Díaz, M.T.;
Goodenough, J.B. SrCo0.95Sb0.05O3-δ as cathode material for high power density solid
oxide fuel cells. Chem. Mater 2010, 22, 789–798.
|