=================================================================================
The shell electrons are bound to atoms by what is called binding energy. Binding energy is also called critical ionization energy. The binding energy of each shell is the minimum energy, which an incoming electron or photon must have to be able to ionize the atom. The strongest binding energy is the K-shell binding energy and the binding energies get weaker for the the further electron from the nucleus. Moreover, the binding energies increase with the atomic number of the nucleus as indicated by the examples in Table 3959.
Table 3959. K-Shell Binding Energy for Various Elements
| Element |
Atomic Number (Z) |
Binding Energy (keV) |
| Oxygen |
8 |
0.5 |
| Calcium |
20 |
4.0 |
| Iodine |
53 |
33 |
| Barium |
56 |
37 |
| Tungsten |
74 |
69.5 |
| Lead |
82 |
88 |
Figure 3959a shows the generation of secondary electrons (SEs) and kinetic energies of the emitted SEs. EKE1 and EKE2 represent the kinetic energies of the two generated SEs. The kinetic energy of the generated SEs is normally in the range of 0 to 50 eV. ΔE1 and ΔE2 represent the energy losses of the incident electrons after the incident electrons interact with the electrons in the K and L3 subshells, respectively. E1 and E2 are the binding energies of the two electrons. E0 is the energy of the incident electrons in the EMs.
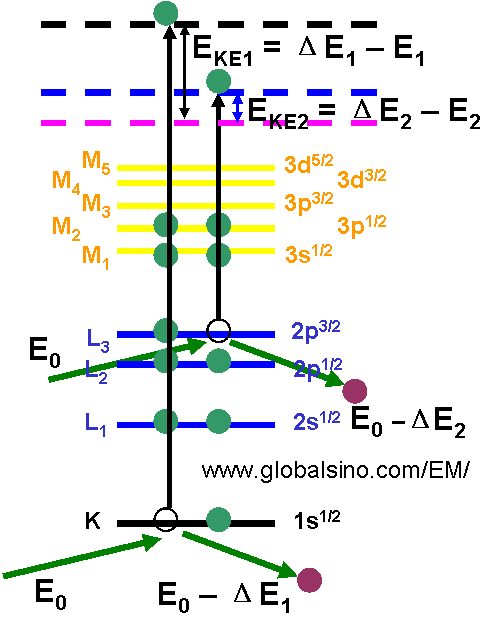
Figure 3959a. The generation of secondary electrons (SEs) and kinetic energies of the emitted SEs.
Figure 3959b shows the relation between electron binding energies, atomic number (Z) and electronic transition. In the figure, the same colors shows the curves and corresponding electronic transitions. Figure 3959c shows the details of the relation between electron binding energies and atomic number (Z) at low binding energies.
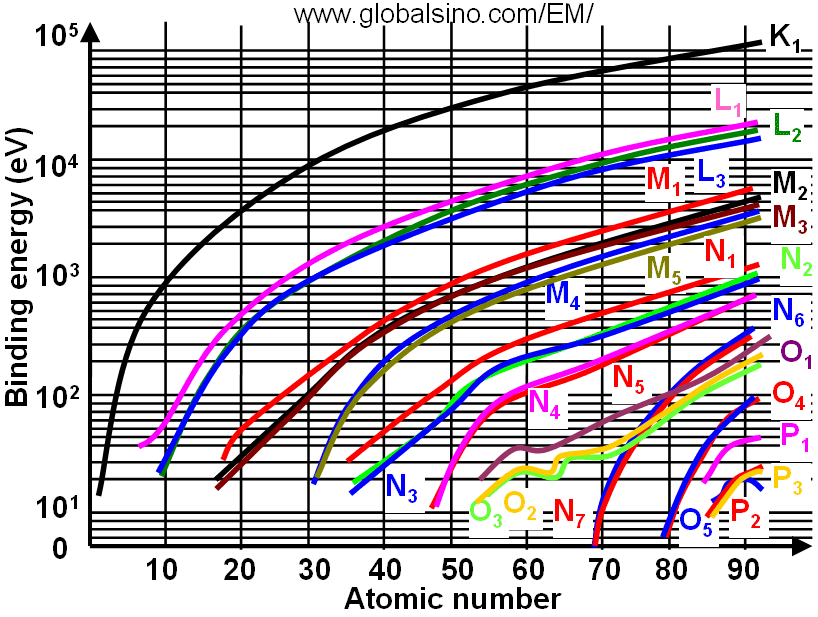
Figure 3959b. Relation between electron binding energies, atomic number (Z) and electronic transition.
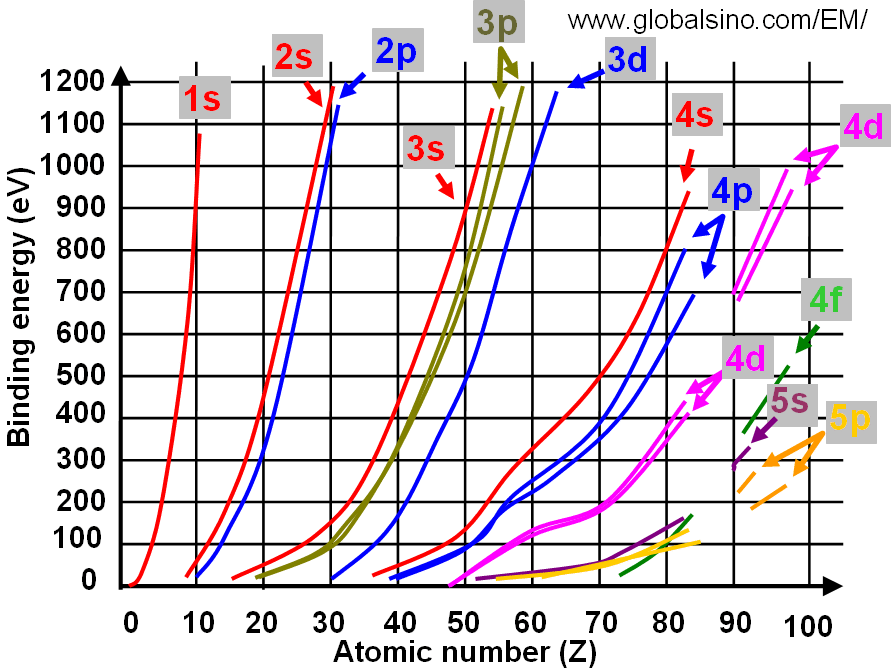
Figure 3959c. Relation between electron binding energies and atomic number (Z) (at low binding energies).
Inner shell excitations occur at energies:
ΔE ≥ EF - EB ----------------------------------- (3959)
where,
ΔE -- The energy loss
EF -- The Fermi level energy
EB -- Binding energy of the inner shell
The EELS feature corresponds to "core loss edges" or "edge onset" (ΔE = EF - EB), see Page 3437.
|