=================================================================================
An electron in the incident electron beam has to transfer an amount of energy greater than a specific value to the inner-shell electron in order to ionize the atom. The minimum energy, which is needed to remove an electron in a shell, is called ionization energy or the critical ionization energy Ec. This threshold energy represents the minimum energy transfer required
for ionization. That is, if we want to generate a useful number of X-rays, then the beam energy E0 must be greater than Ec. Ec is greater if the electrons are more tightly bound to the nucleus. For a specific element, the innermost shell (K) has a higher Ec than the next shell (L), and so on. Atoms with higher Z have more protons and thus, the core electrons are bonded to the nucleus more tightly, having a higher Ec.
Critical ionization energy is also known as absorption edge energy, critical excitation energy, or X-ray absorption energy. It is higher than the associated characteristic (line) X-ray energy. The EELS edge onset is the sudden rise in intensity preceding peaks, representing the ionization threshold which approximately corresponds to the inner-shell binding energy.
Figure 4671 shows the schematic illustration of the energy level diagrams for molecular structures with different number of atoms which are single atoms, dimers, clusters and bulk materials. Splitting of the atomic energy levels occurs when the single atoms form a diatomic molecule. As more atoms join the system, the levels split further until a quasi-continuous band structure is formed in the bulk material. In other words, quantum size effects occur when the quasi-continuous band structure of a solid state system begins to break down as more atoms are included.
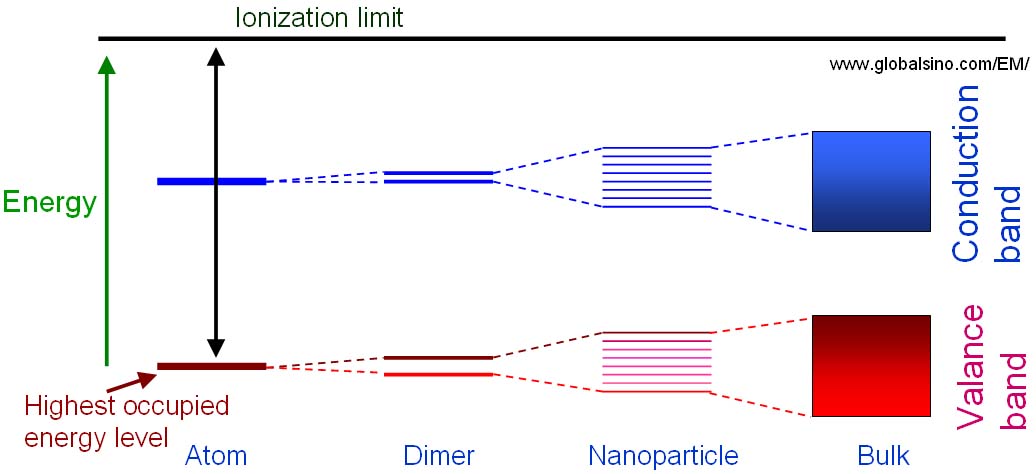
Figure 4671. Schematic illustration of the energy level diagrams for molecular structures with different number of atoms which are single atoms, dimers, clusters and bulk materials.
|