EELS Measurement of Lithium Element - Practical Electron Microscopy and Database - - An Online Book - |
|||||||||
| Microanalysis | EM Book https://www.globalsino.com/EM/ | |||||||||
As shown in Table 2649, because the Li-K edge situates right after plasmon peaks, substraction of the background using a standard power-law model becomes extremely difficult. Table 2649. Main edges of Li used in EELS analysis.
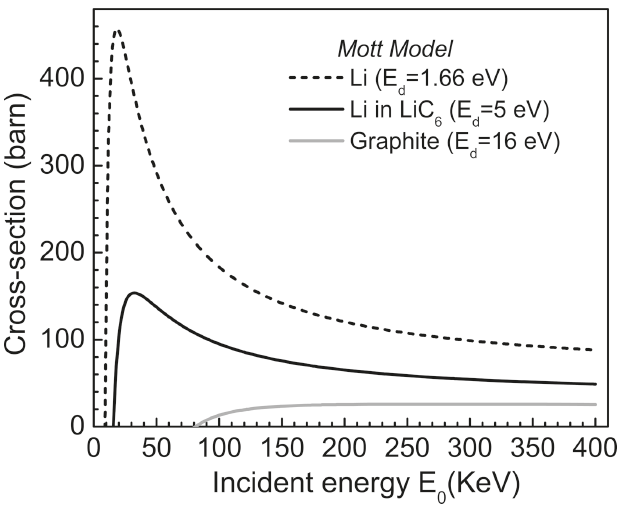
Electron energy loss spectroscopy (EELS) in TEM is applicable to detect and map lithium because of the high ionization cross-section of the shallow Li K-edge that is 10-100 times greater than those of other light elements, e.g. O and F. However, probing lithium is extremely challenging with STEM techniques due to its vulnerability to radiation damage. Conventional wisdom suggests that a low operating voltage (<100 kV) in TEMs is optimal for reducing radiation damage, but the calculated profiles as a function of incident electron energy E0 in Figure 2649a demonstrate the minimum displacement cross-sections for lithium atoms in the elemental Li, lithiated graphite (LiC6) and graphite are not at low operating voltages.
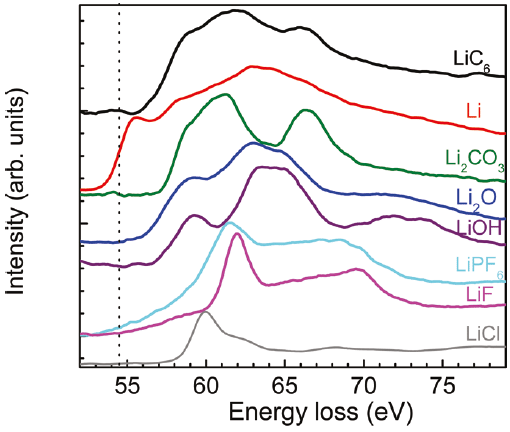
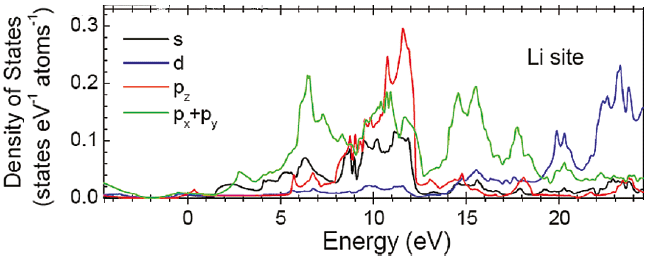
Figure 2649a. The displacement cross-sections for lithium atoms in the elemental Li, lithiated graphite (LiC6) and graphite as a function of incident electron energy E0. Ed is the displacement energy. [1] Plural inelastic scattering by plasmon excitation is a special concern when measuring the low energy loss (e.g. Li K-edge) because it is close to the low-energy plasmon region. For instance, double plasmon scattering distorts the pre-edge background and can mask the Li K-edge. Artifacts due to plural scattering can be reduced by increasing the inelastic mean-free-path with the increase of the accelerating voltage of the electron beam or by restricting analyses to thin regions of the sample. Figure 2649b shows the Li K-edge spectra of elemental Li and some Li compounds. A chemical shift of about 2.7 eV, with respect to elemental Li, was observed in the Li K-edge from LiC6. As shown in the calculated electronic structure using Wien2k code in Figure 2649c, the Li PDOS (projected density of states) is dominantly contributed by the 2p and 3s states within 20 eV of EF.
Figure 2649c. Li PDOS is dominantly contributed by the 2p and 3s states within 20 eV of EF. Adapted from [1]
[1] FengWang, Jason Graetz, M. Sergio Moreno, Chao Ma, LijunWu, Vyacheslav Volkov, and Yimei Zhu, Chemical Distribution and Bonding of Lithium in Intercalated Graphite: Identification with Optimized Electron Energy Loss Spectroscopy, ACS Nano, 5 (2), (2011) 1190.
|
|
||||||||