=================================================================================
An atom is ionized when an inner shell electron is removed by high-energy-electron radiation. To return the excited atom to its ground state, the electron from an outer, higher energy shell fills the vacant inner shell, and then the atom releases an amount of energy equal to the potential energy difference between the two shells. This excess energy, which is unique for every atomic transition, will be emitted by the excited atom either as an X-ray photon (for EDS measurement: Energy Dispersive X-ray Spectrum) or will be self-absorbed and emitted as an Auger electron (for AES measurement: Auger Electron Spectrum).
Auger electrons can be generated with the probes of incident x-rays or charged particles. In these cases, Auger electron spectroscopy (AES) can be performed. The highest spatial resolution in AES can be obtained by using an electron beam as the probe. Similar to EELS and EDS, Auger peak energies are characteristic of each element presenting in the sample. Figure 3926 shows both generation processes of x-rays and Auger electron.
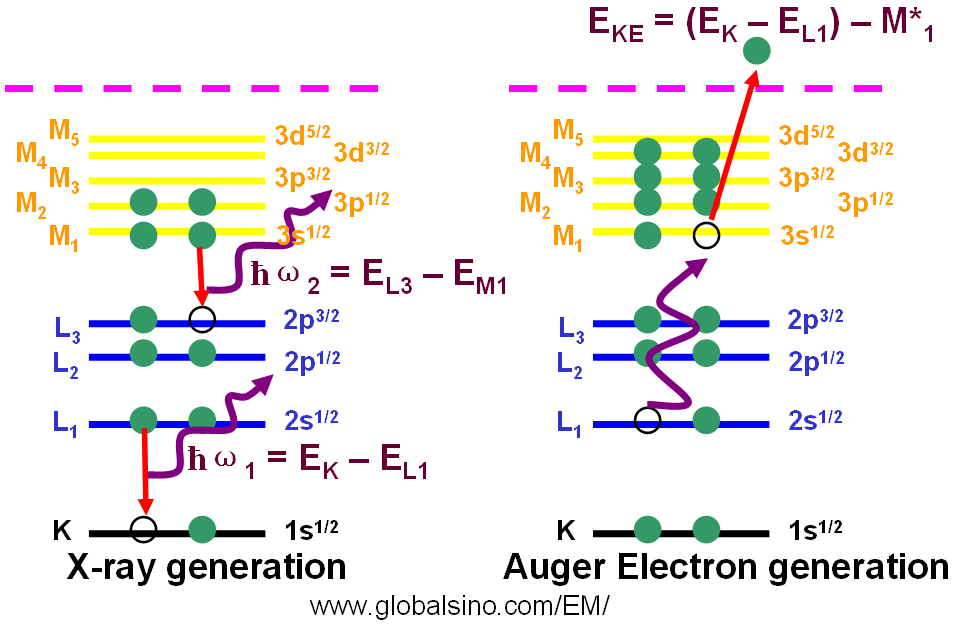
Figure 3926. Generation processes of x-rays and Auger electron.
For insulators, due surface charging, the surface potential is inherently stabilized at an equilibrium positive value, and thus a uniform shift (typically
2–10 eV) of the Auger spectrum to lower energy is induced.
|