Mass Absorption Coefficients of X-Rays - Practical Electron Microscopy and Database - - An Online Book - |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Microanalysis | EM Book https://www.globalsino.com/EM/ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ================================================================================= | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
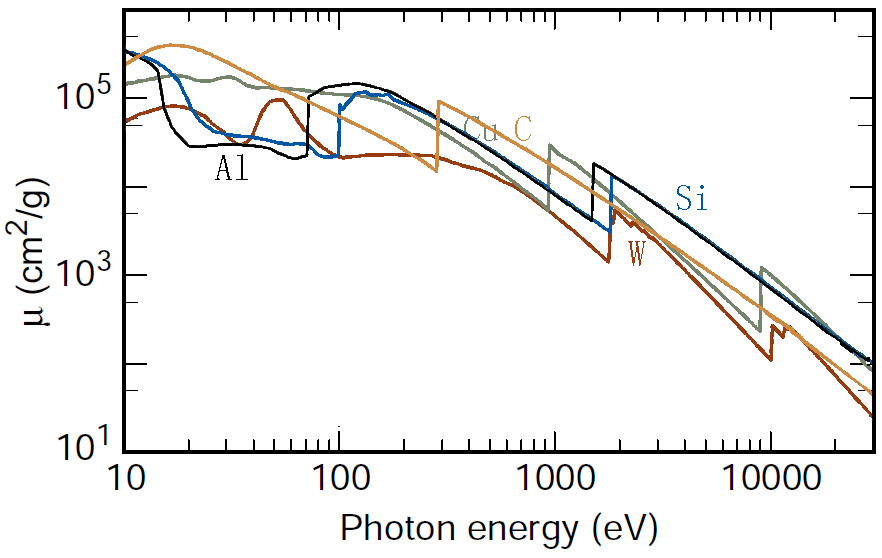
The intensity of the transmitted X-ray is a function of the intensity of a generated X-ray (I0), mass absorption coefficient μ/ρ (cm2/g), material density ρ (g/cm3), and thickness (cm): As an example, page1730 shows X-ray absorption and intensity reduction induced by carbon contamination in EMs. Equation 1313a can be re-written as, Assuming all X-rays, at different wavelengths, penetrating the same materials through the same thickness, e.g. X-rays collected by EDS detector in TEM or SEM systems, then the collected X-ray intensities decreases experientially depending on the corresponding mass absorption coefficient as indicated by Equation 1313b and Figure 1313a.
Figure 1313a. . Table 1313. Mass absorption coefficients (μ/ρ, cm2/g) of several elements.
The mass absorption coefficient (e.g. Figure 1313b) can be given by,
The corresponding mass absorption coefficient of a compound can be calculated by the equation, Note that the mass absorption coefficient for a mixture can also be calculated in the same way as shown in Equation 1313c.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ================================================================================= | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
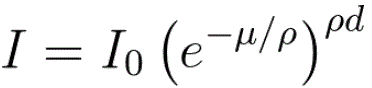 -------------------------------------- [1313b]
-------------------------------------- [1313b]