=================================================================================
The wurtzite structure, named after the mineral wurtzite, is a crystalline structure for various binary compounds. This structure is a branch of a hexagonal crystal system.
For instance, the atoms in nitride semiconductors are tetrahedrally coordinated with hybrid sp3 orbitals. As shown in Figure 2080, the atomic layers of the nitride semiconductors are arranged in hcp (hexagonal closed packed) planes with alternating sub-layers consisting of Group III and V elements. These layers can be stacked in different ways. For instance, Figure 2080 (a) and (b) shows a configuration in the sequence …ABABAB…, resulting in the hexagonal wurtzite structure (space group P63mc), while Figure 2080 (c) and (d) shows a stacking sequence of the type... ABCABCABC… , resulting in the cubic zincblende crystal structure (space group F-43m). The indices of the stacking planes are {0001} for the hexagonal and {111} for the cubic structures. One example is that GaN cubic zincblende crystal structure can transform into hexagonal wurtzite phase due to annealing.
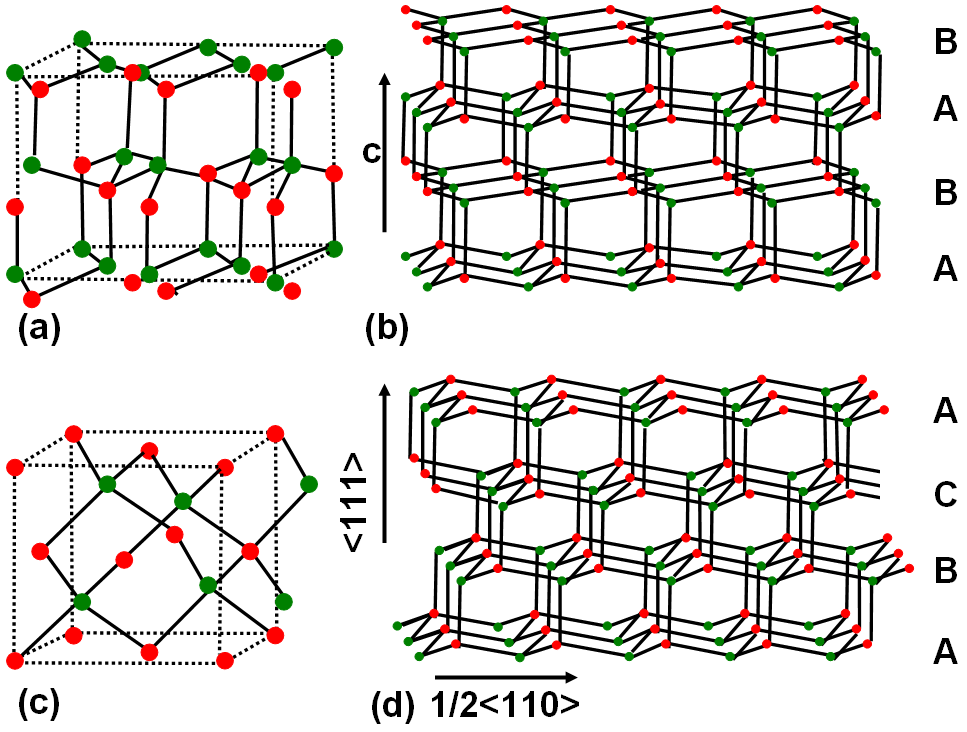
Figure 2080. Two main types of atomic configurations in the nitride semiconductors. Unit cells for (a) hexagonal wurtzite structure (b) cubic zincblende structure, and (b) and (d) their corresponding ctystalline layer stacks, respectively.
Table 2080a. Examples of Wurtzite structures.
Crystals |
Lattice constant (nm) |
a |
b |
AlN |
0.311 |
0.498 |
BeO |
0.270 |
0.438 |
CdS |
0.413 |
0.675 |
CdSe |
0.430 |
0.702 |
CuH |
0.289 |
0.461 |
MgTe |
0.452 |
0.733 |
NH4F |
0.439 |
0.702 |
SiC |
0.308 |
0.505 |
ZnO |
0.325 |
0.523 |
ZnS |
0.381 |
0.623 |
Table 2080b. Other characteristics of Wurtzite Structures.
|