Yield of Auger-Electrons - Practical Electron Microscopy and Database - - An Online Book - |
||||||
| Microanalysis | EM Book https://www.globalsino.com/EM/ | ||||||
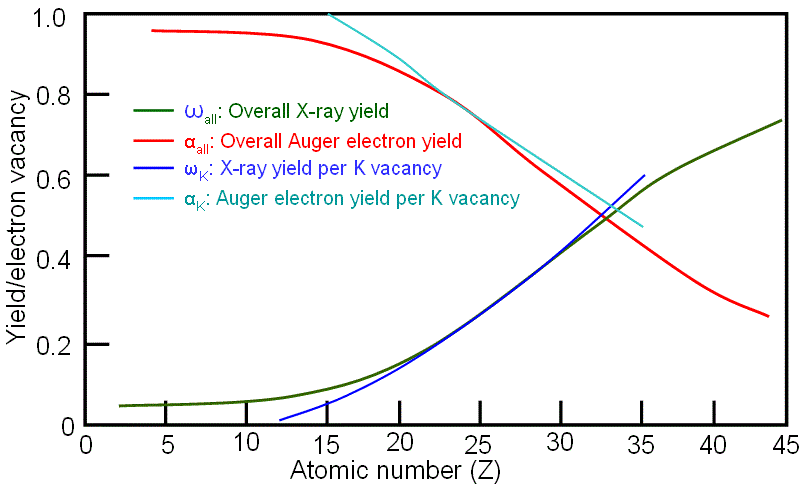
An atom is ionized when an inner shell electron is removed by high-energy-electron radiation. In this step, vacancies in inner shell are formed. To return the excited atom to its ground state, the electron from an outer, higher energy shell fills the vacant inner shell, and then the atom releases an amount of energy equal to the potential energy difference between the two shells. This excess energy, which is unique for every atomic transition, will be emitted by the excited atom either as an X-ray photon (for EDS measurement: Energy Dispersive X-ray Spectrum) or will be self-absorbed and emitted as an Auger electron (for AES measurement: Auger Electron Spectrum). Auger electrons and X-rays are the only possible outcomes, and the sum of the two probabilities is unity. The rate of Auger electron emission includes two electrons, and is evaluated for a Coulomb interaction between them. From the discussion on page4637, we know that heavy elements tend to emit X-rays and light elements tend to emit Auger electrons. As an example, Figure 2354a shows the X-ray and Auger electron yields per K vacancy as a function of atomic number as well as from all vacancies generated by accelerated incident electrons. For low-Z material Auger emission is dominant, while for high-Z material X-ray emission dominates. The two emission probabilities are approximately equal at Z ≈ 30 for K shell ionization.
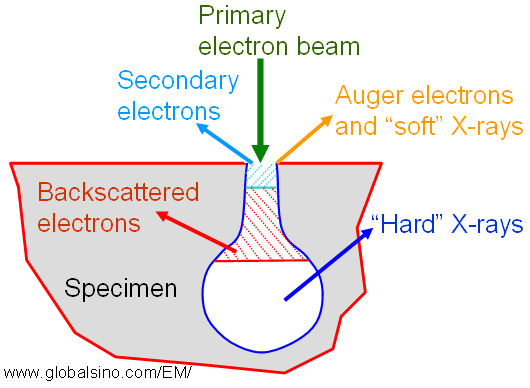
The relationship between the yields of K X-ray lines (ωK) and K Auger electrons (αK) per K vacancy is given by, Figure 2354b shows the escape depths of various species generated by high-energy electrons penetrating into a solid. Auger electrons and "soft" X-rays are emitted in depth of <1 nm and in diameter of beam size, secondary electrons are emitted in depth of < 10 nm and in diameter of beam size, and the emission depths and diameters of backscattered electrons and "hard" X-rays depend on both the beam energy and beam size.
Figure 2354b. Escape depths of various species generated by high-energy electrons penetrating into a solid.
In comparison between the emissions of Auger electrons and “soft” X-rays, for electron irradiation of high atomic-number (Z) elements, Auger electron emission predominates, while for irradiation of low Z elements, “soft” X-rays predominate.
|
|
|||||