=================================================================================
The electrons of an isolated atom can be separated into two groups:
i) Valence electrons.
ii) Core electrons.
The core electrons are mostly localized around the nuclei in the completely filled inner atomic shells. The valence electrons are in the outermost, partially filled atomic shells. The main properties of materials are determined by the valence electrons rather than by the core electrons. For this reason, the inner shell electrons are neglected in many analyses.
Figure 2375 shows an example of the ionization processes and generations of X-rays. A high-energy electron (incident electron) must penetrate through the outer conduction/valence bands and interact with the inner-shell (or core) electrons. If the high-energy electron transfers more than a critical amount of energy to an inner-shell electron (K electron here), that electron is ejected into the vacuum, that is, it escapes (step 2b in the figure) the attractive field of the nucleus, leaving a hole in the inner shell (K shell in the figure) and escapes above the Fermi level into the unfilled states. In this case, the atom is ionized. The excited atom can return almost to its ground state (lowest energy) by filling in the hole with an electron from an outer shell (step 3). This transition is accompanied by the emission of an X-ray in Figure 2375. The energy of the X-ray emission is characteristic of the difference in energy between the two electron shells involved (L3 → K in the figure) and this energy difference is unique to the specific atom.
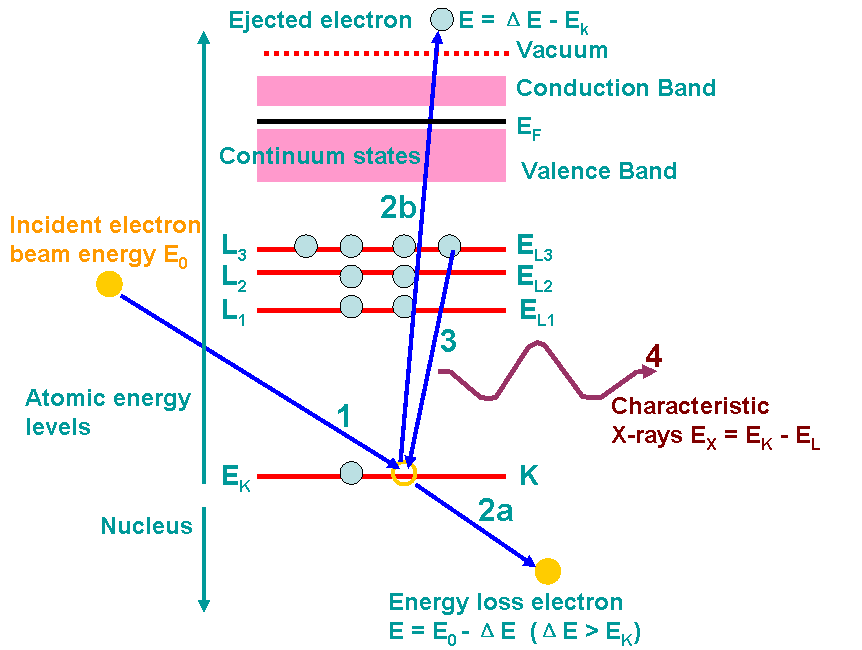
Figure 2375. Example of the ionization processes and generations of X-rays. The numbers indicates the process sequence. In this process, an inner (K) shell electron is ejected
from the atom by a high-energy electron (incident electron). In step 3, the hole in the K shell
is filled by an electron from the L shell (L3 here), characteristic (Kα) X-ray emission
occurs (step 4). The beam electron loses energy but continues on through the
specimen (step 2a). Steps 2a and 2b almost occur at the same time.
Table 2375. Comparison between outer (valence) and inner (core) electrons.
|
Outer electrons |
Inner electrons |
Principal quantum number |
Correspond to the highest value of principal quantum number in the atom |
Correspond to the lowest values of the principle quantum number |
Electron shell |
|
Occupy completely filled shells |
Distance from nucleus |
Are on average farther from the nucleus than other electrons |
Are on average closer to the nucleus than other electrons |
Binding energy of electron |
Are the least strongly bound electrons |
The highest binding energy |
Move out from their positions |
Are easily moved from their positions |
Are not easily moved from their positions near the nuclei |
Chemical reactions |
Originate from the exchange of electrons from the outer electronic shell of atoms |
The most inner shells do not participate in chemical reactions due to the high electrostatic attraction to the nucleus |
Chemical bonds between atoms |
Because of electrons from the outermost shell |
Not related |
Chemical bond formation |
The energy of valence electrons is directly modified by the bond formation process |
Do not directly contribute to the chemical bond formation |
Electrical conduction |
Directly participate |
Do not directly participate |
Energy change |
The energy of valence electrons is directly modified by the bond formation process |
Their energies are affected by the corresponding changes in the valence charge distribution |
Localization of electronic states |
Form delocalized electronic states that are characterized by three-dimensional wavefunctions known as Bloch waves |
Are strongly localized near the nuclei and do not form bands. |
EELS background is formed mainly due to the valence-electron contribution, and it can be subtracted by a least-squares
fit to an E-r dependence at lower energy loss. [1]
Page3375 lists the behaviors and properties of various inelastic electron scatterings in electron interaction with materials, including inter- and intra-band transitions, inner shell ionization, phonon excitation and plasmon excitation.
[1] R. F. Egerton, K-shell ionization cross-sections for use in microanalysis, 4(2), (1979) 169-179.
|