=================================================================================
Figure 2495 shows the XRD JCPDS (Joint Committee on Powder Diffraction Standards) card of sodium chloride (NaCl). The meaning of ➀ to ➈ is described on page2496.
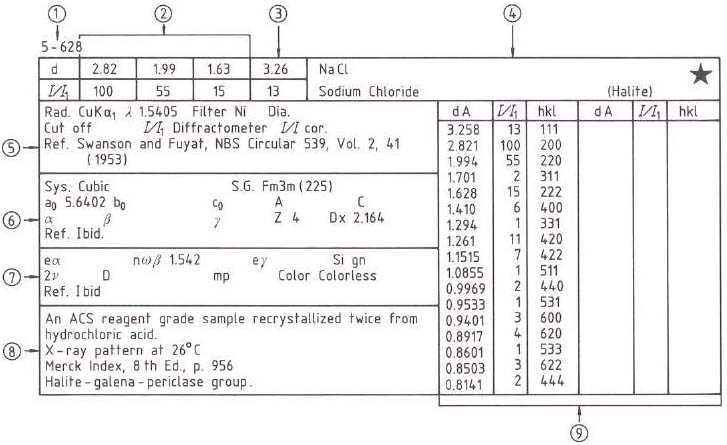
Figure 2495. The XRD JCPDS card of NaCl. The star at the top-right corner indicates the quality of the data.
As listed in Table 2495a, substances with large bonding energies usually have high melting temperatures.
Table 2495a. Bonding energies and melting temperatures of ionic substances.
Bonding type |
Substance |
Bonding energy |
Melting point (°C) |
| kJ/mol |
kcal/mol |
eV/Atom, Ion, or Molecule |
Ionic
|
Typical value |
50-1000 |
|
|
|
| NaCl |
640 |
153 |
3.3 |
801 |
| MgO |
1000 |
239 |
5.2 |
2800 |
Table 2495b. Other characteristics of Burgers vectors.
| Characteristics |
|
| Dominating slip planes, slip directions and stable Burgers vector for common crystal structures |
page3557 |
| Tables of Burgers vectors of dislocations and g·b |
page1995 |
Table 2495c. Crystals with sodium chloride structures.
Crystal |
Lattice constant (a, nm) |
| AgBr |
0.577 |
| AgCl |
0.555 |
| AgF |
0.492 |
| BaO |
0.552 |
| BaS |
0.639 |
| BaSe |
0.660 |
| BaTe |
0.699 |
| CaS |
0.569 |
| CrN |
0.414 |
| CsF |
0.601 |
| FeO |
0.431 |
| KBr |
0.660 |
| KCl |
0.630 |
| KF |
0.535 |
| KI |
0.707 |
| LiBr |
0.550 |
| LiCl |
0.513 |
| LiF |
0.402 |
| LiH |
0.409 |
| LiI |
0.600 |
| MgO |
0.421 |
| MgS |
0.520 |
| MgSe |
0.545 |
| MnO |
0.444 |
| MnS |
0.522 |
| MnSe |
0.549 |
| NaBr |
0.597 |
| NaCl |
0.564 |
| NaF |
0.462 |
| NaI |
0.647 |
| NiO |
0.417 |
| PbS |
0.593 |
| PbSe |
0.612 |
| PbTe |
0.645 |
| RbBr |
0.685 |
| RbCl |
0.658 |
| RbF |
0.564 |
| RbI |
0.734 |
| SnAs |
0.568 |
| SnTe |
0.631 |
| SrO |
0.516 |
| SrS |
0.602 |
| SrSe |
0.623 |
| SrTe |
0.647 |
| TiC |
0.432 |
| TiN |
0.424 |
| TiO |
0.424 |
| VC |
0.418 |
| VN |
0.413 |
| ZrC |
0.468 |
| ZrN |
0.461 |
|