=================================================================================
Most perovskite crystals are oxides with the general formula ABO3, where A and B represent two different cations and O represents oxygen elements. The A-site cations are larger than B-site cations. Most perovskite structures do not have such ideal cubic symmetry and are normally distorted, even for the prototype perovskite, CaTiO3. For instance, the properties of the cations on the A-sites and on the B-sites often induce common distortions such as cation displacements within the octahedra and tilting of the octahedra. In general, the degree of distortion in ABO3 perovskites can be determined by [1],
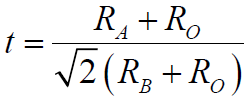 ----------------------------- [3531] ----------------------------- [3531]
where,
RA, RB and RO -- The ionic radii of the A-site cation, B-site cation and oxygen anion, respectively
t -- Goldschmidt tolerance factor
In an ideal cubic perovskite crystalline structure the A-site and B-site cations optimize their equilibrium bond distances to the oxygen elements without inducing any distortion of the unit cell at t = 1. When 0.78 < t < 1.05, a distorted perovskite structure can normally be stabilized.
[1] V. M. Goldschmidt, T. Barth, G. Lunde, and W. Zachariasen, Skrifter Norske Videnskaps-Akad. Oslo, Mat.-Nat. Kl. 2, 117 (1926).
|
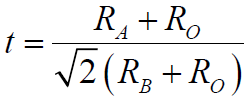 ----------------------------- [3531]
----------------------------- [3531]