|
This book (Practical Electron Microscopy and Database) is a reference for TEM and SEM students, operators, engineers, technicians, managers, and researchers.
|
=================================================================================
In order to make more easily described mathematically, a non-primitive hexagonal unit cell is always used to express a rhombohedral lattice by adopting the axes a, b, and c indicated by the blue arrows in Figure 3546a, which has the cell transferred to one centered at the points 1/3, 2/3, 2/3 and 2/3, 1/3, 1/3 and thus, the cell is three times as large, but has the shape of the conventional hexagonal cell (in plum) with the c-direction as the unique axis. The lattices which have rhombohedral centering are normally given the symbol R.
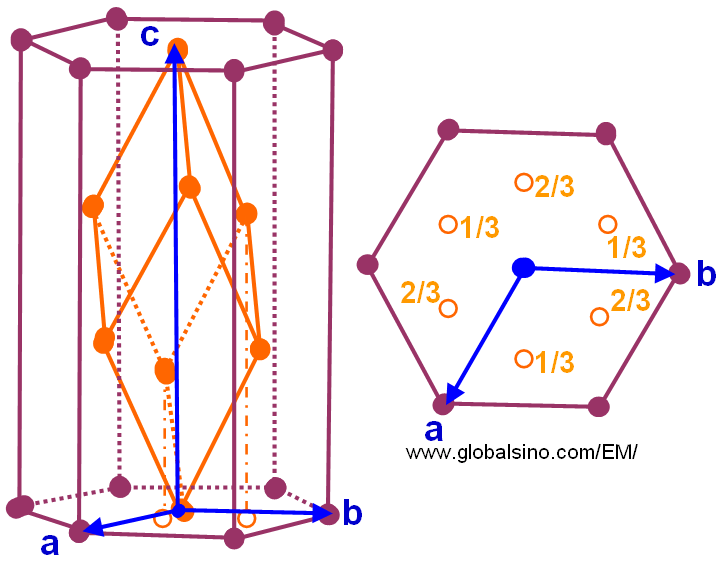
Figure 3546a. Relationship between the hexagonal and primitive rhombohedral unit cells.
For instance, sapphire (α-Al2O3) as a hexagonal structure, belonging to the space group R3c, can be expressed both as a hexagonal as well as a rhombohedral unit cell. The basic structure consists of hexagonal close-packed planes of oxygen intercalated with aluminum planes. As shown in Figure 3546b, the aluminum planes have a similar hexagonal close-packed arrangement but with 1/3 of the sites vacant, resulting in an Al/O ratio of 2/3. Each Al atom is coordinated by 6 oxygen atoms, and each oxygen atom has 4 Al neighbours. The vacancies are aligned on the so-called R-planes, giving sapphire the ability to cleave along these rhombohedral planes. Due to these properties, the unit cell of sapphire is chosen taking into consideration of the position of the A1 vacancies.
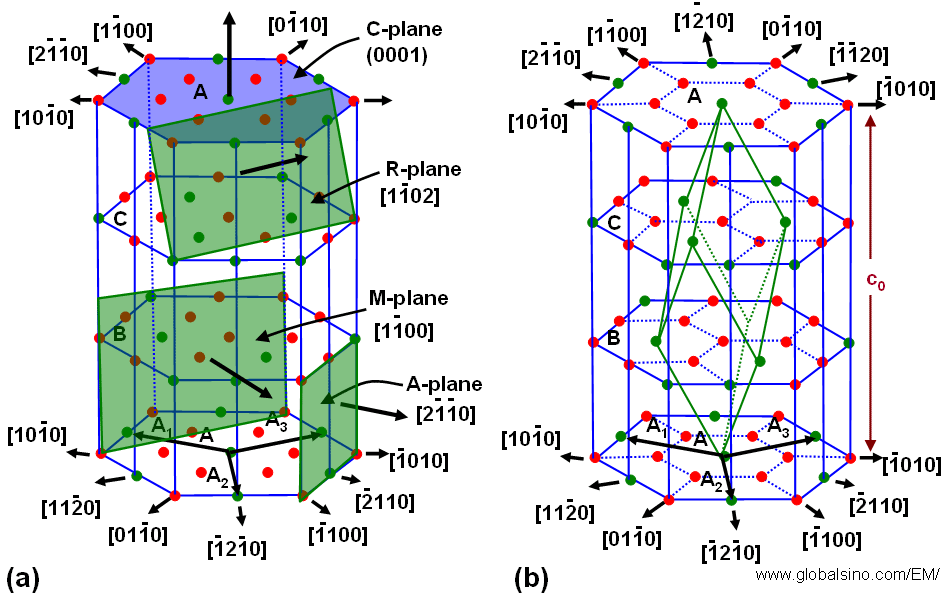
Figure 3546b.The crystal lattice of sapphire. (a) A-, M-, R-, and C-planes of the crystal; (b) Schematic illustration of the aluminum planes. Oxygen planes are intercalated with the aluminum planes (not shown).
|

