=================================================================================
The superlattices of two perovskites SrTiO3 and LaTiO3have many important properties of naturally occurring charge-ordered systems, i.e. mixed-valence configurations near half-filling. Their lattice constants (see SrTiO3 and LaTiO3) are relatively well matched, and the continuity of the TiO6 octahedral lattice across the superlattice minimizes the perturbation of the electronic states near the chemical potential [1-2].
For perovskite cell structures, octahedral oxygen (O) bonding around the atom (Mn for LaMnO3 as shown in Figure 3518a) in B-site generates the crystal field around the B-site atom. The valence states of the atoms (e.g. Mn) are fixed by charge neutrality. In the case of LaMnO3, if the parent compound is doped with Sr2+ for La3+, then Mn3+ (d3) is replaced by Mn4+ in the lattice.
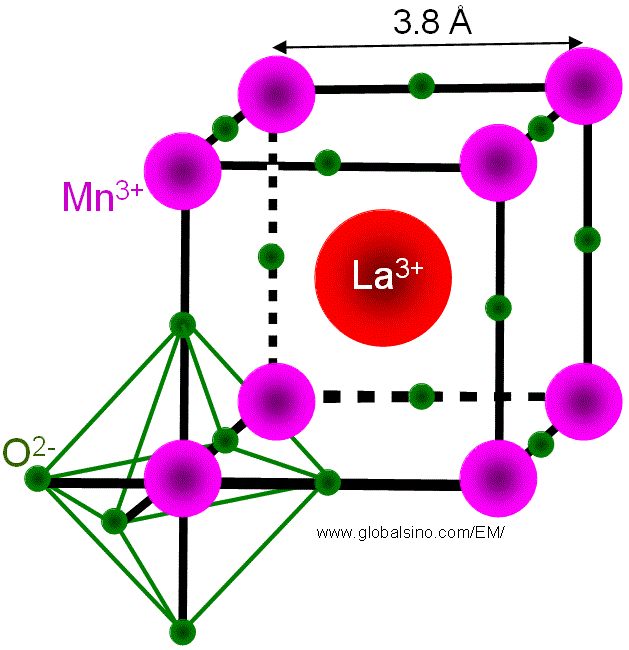
Figure 3518a. Octahedral oxygen bonding around the Mn atom in LaMnO3.
Figure 3518b shows the molecular orbital energy structure for an octahedral TMO6 cluster. The split t2g and eg orbitals can only mix with symmetry adapted linear combinations of the 6 oxygen (O) 2p orbitals having the same symmetry. The separation between the t2g and eg levels is called the LF (ligand field) splitting parameter ΔO. σ-overlap is larger than π-overlap so that the upward shift of the eg level is larger than the upward shift of the t2g level.
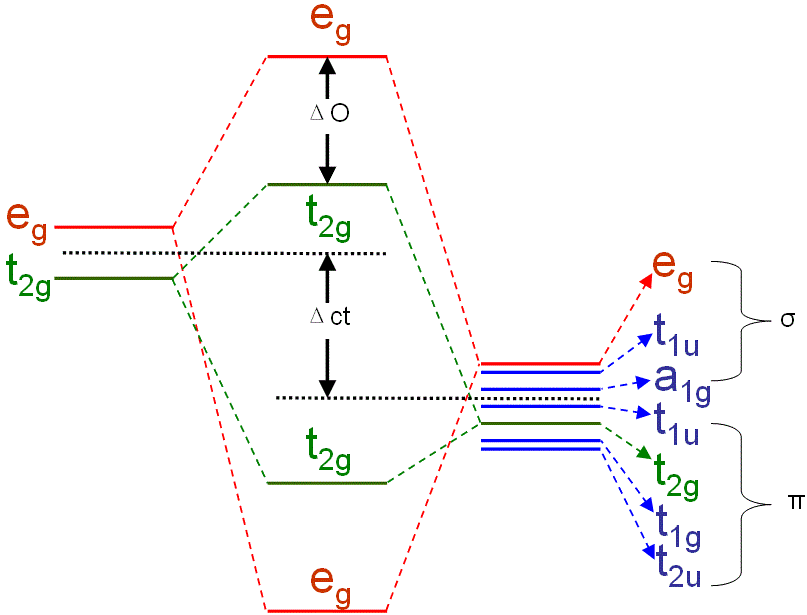
Figure 3518b. Molecular orbital energy structure for an octahedral TMO6 cluster.
As shown in Figure 3518c, in a cubic transition-metal (TM) oxide crystal, the divalent TM ion is in a site that has octahedral Oh symmetry and the d-levels split into threefold degenerate (lower energy) t2g states and twofold degenerate (higher energy) eg states that can accommodate six and four electrons, respectively (including spin states). In this case, these metal d-orbitals are split by an octahedral crystal potential. The tetragonal symmetry splits the levels further. The t2g states split into a singlet, dxy, and a doublet dxz and dyz. The eg states split into d3z2-r2 and dx2-y2 levels.
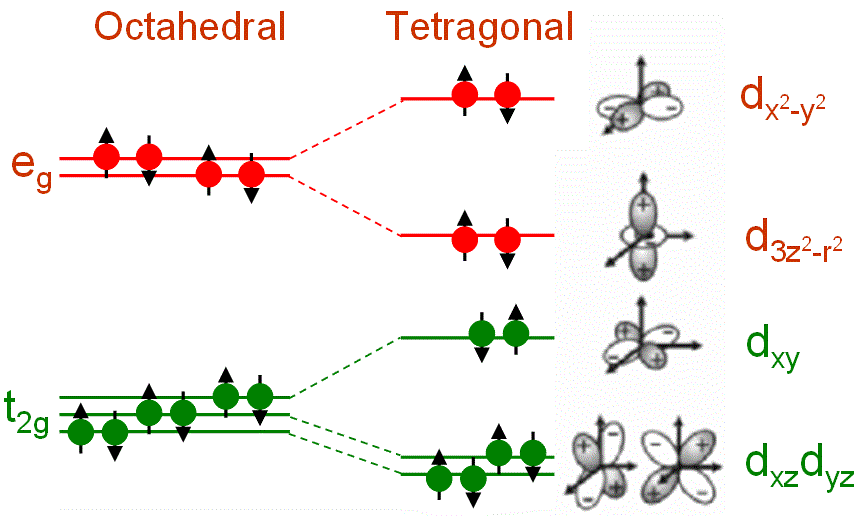
Figure 3518c. Ligand field splitting of d orbitals in an octahedral ligand field.
Figure 3518d shows the ligand field splitting of d orbitals for Fe in an octahedral ligand field, resulting in lower energy t2g and higher energy eg orbitals and presenting the spin states of Fe in octahedral field. Two cases are show on the right of the figure. If an exchange energy larger than the t2g - eg splitting favors parallel spin alignment, then a high spin ground state is obtained. If the exchange energy is smaller than the t2g - eg splitting, then a low spin ground state is obtained. In this case, the fourth and fifth electrons are not in the high-energy eg state but are located in the lower t2g state.
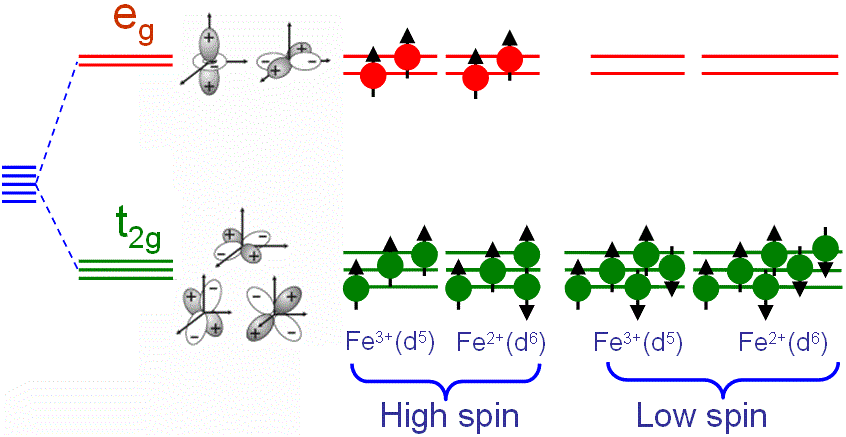
Figure 3518d. Ligand field splitting of d orbitals for Fe in an octahedral ligand field.
In some TM (transition metal) cases, the filling of orbitals with electrons may affect the local structure and thus induce geometrical distortion around the TM ion. The Jahn–Teller effect, also called Jahn–Teller distortion, describes this type of distortions. A typical Jahn-Teller ion is Mn3+ as shown in Figure 3518e. The ion in the high-spin configuration contains a single electron in the upper eg state when it is placed in an octahedral LF (ligand field). A tetragonal distortion can lower the energy of the system. The lowering in total energy is due to the lowering of one of the eg orbitals by lengthening the bond along the z axis. Note that the overall energy of the system is not further lowered by splitting the t2g state because the center of gravity is retained.
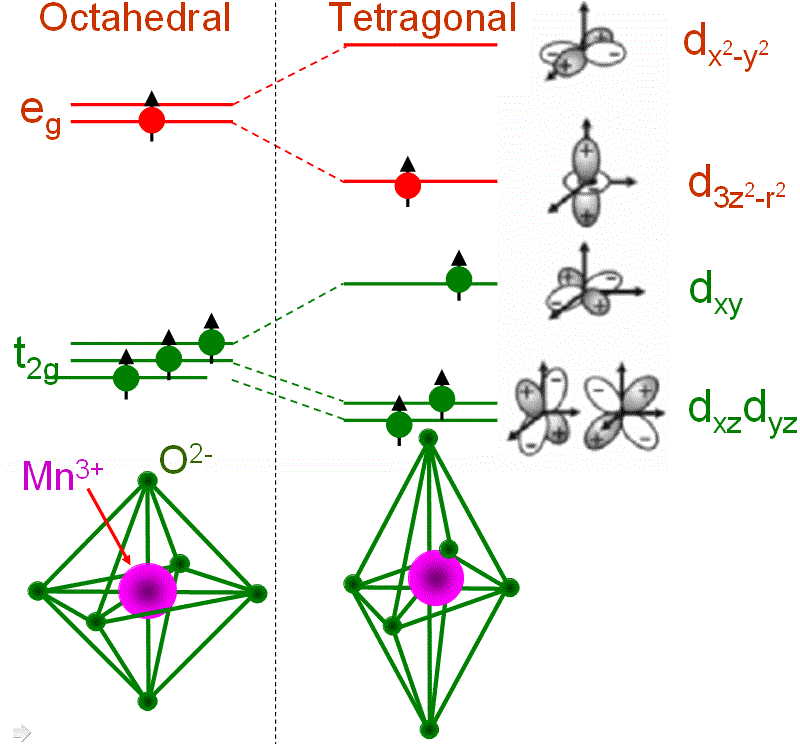
Figure 3518e. John-Teller effect for Mn3+ (3d4).
[1] Sunstrom, J. E. IV, Kauzlarich, S.M. & Klavins, P. Synthesis, structure, and properties of La1-xSrxTiO3 (0 ≤ x ≤ 1). Chem. Mater. 4, 346–353 (1992).
[2] Kahn, A. H. & Leyendecker, A. J. Electronic energy bands in strontium titanate. Phys. Rev. 135, A1321–A1325 (1964).
|