Analysis of White Lines in EEL Spectrum - Practical Electron Microscopy and Database - - An Online Book - |
|||||
| Microanalysis | EM Book https://www.globalsino.com/EM/ | |||||
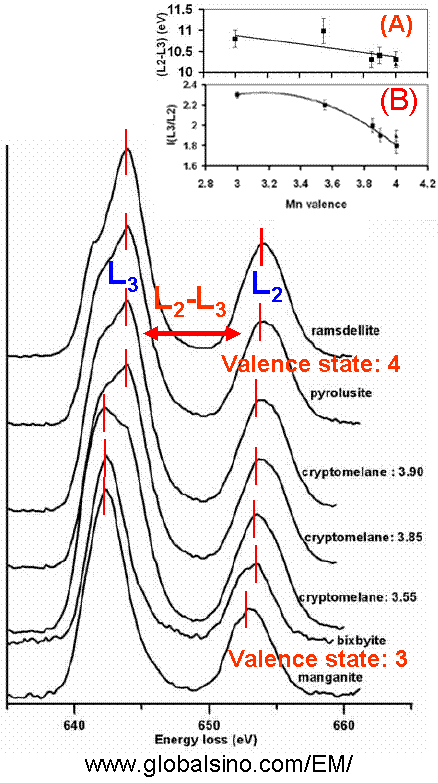
The two strong L3 and L2 white lines originate from the transition of electrons from the spin-orbit split levels 2p3/2 and 2p1/2 to unoccupied 3d states. The L3/L2 ratio increases as the oxidation states of transition metals decrease and thus can be used to determine the oxidation states of cations, for instance, in 3d transition metal-oxides. As an example, Figure 4337 shows the EELS profiles of ramsdellite, pyrolusite, cryptomelane (for different valence states), bixbyite, and manganite materials. Various methods can be performed to understand the chemical properties of the elements in compounds, based on analysis of EELS white lines.
Figure 4337. The EELS profiles of ramsdellite, pyrolusite, different cryptomelane (synthesized Edges shift Figure 4337 shows that the two Mn L2,3-edges shift to higher energy loss range with the increase of oxidation state. Width of the edge of the white lines depending on valence states Figure 4337 shows that the Mn L3-edge structure of Mn3+ compounds is narrower than the edges for the Mn4+ valence states. Width of the edge of the white lines depending on material structure Indicated in Figure 4337, unlike Mn3+ compounds, different structural types of MnO2 (Mn4+ valence states) produce different widths of the Mn L3 edge. The main L3 peak from ramsdellite is apparently narrower than that of pyrolusite [2] because various arrangements of Mn octahedra give rise to different shapes of their EELS spectra [3]. Symmetry of the white line edge Figure 4337 shows that the Mn L3-edge structure of Mn3+ compounds is more symmetric than the edges for the Mn4+ valence states. Energy difference between the two peaks of the white lines The inset (A) in Figure 4337 shows the energy difference (ΔE(L2-L3)) between the two peaks of L2,3-edges, corresponding to the different valence states and indicating a linear trend but with large errors as a result of the Coster-Kronig Auger decay effect on the broadening of Mn L2 edges. Intensity ratio of the two peaks of the white lines The inset (B) in Figure 4337 shows the integrated Mn L2,3 white-line intensity ratios, I(L3)/I(L2), as a function of the Mn valence state. The white-line intensities were integrated over 4 eV windows centered at the L2,3 peak maxima.
[1] Shouliang Zhang, Kenneth J.T. Livi, Anne-Claire Gaillot, Alan T. Stone, and David R. Veblen, Determination of manganese valence states in (Mn3+, Mn4+) minerals by electron energy-loss spectroscopy, American Mineralogist, 95(11-12) 1741-1746. |
|
||||