=================================================================================
The tetragonal tungsten bronze (TTB) is designated after the tetragonal potassium tungsten bronze KxWO3 [1]. This structure consists of a framework of corner-sharing MO6 octahedra in which different tunnels are oriented along the short crystallographic axis (c axis). As shown in Figure 3352a when projected onto the ab plane, three distinct types of tunnels appear, which have trigonal, square or pentagonal shapes. The TTB structure can be geometrically derived from ReO3 type either by a rotational shear operation involving the central four octahedral of a block of 4 x 4 octahedra [2] or by a “fourling” (four-fold twinning ) operation [3]. The possibility to incorporate various cations into the square and pentagonal tunnels contributes to the versatility of TTB-type phases [4].
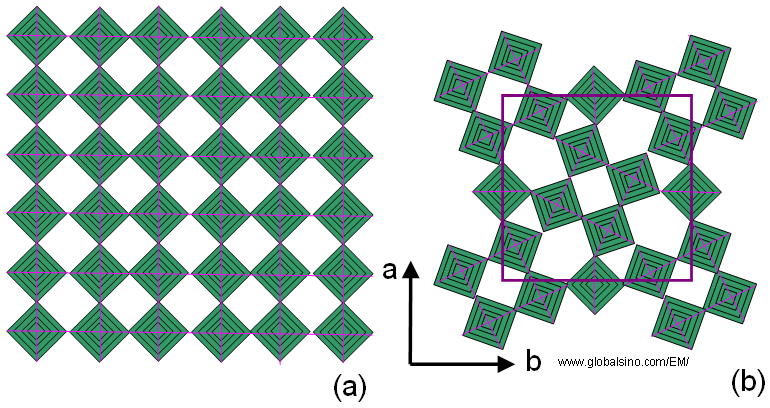
Figure 3352a. Schematic illustration of (a) Ideal ReO3 type and (b) Tetragonal
tungsten bronze (TTB) type projected onto the ab-plane.
As an example, Figure 3352b shows the average lead-TTB structure (e.g. PbxNb1.17W1.0O5.93+x) obtained by XRD technique and projected onto the ab plane. Note that only the main lead positions are shown.
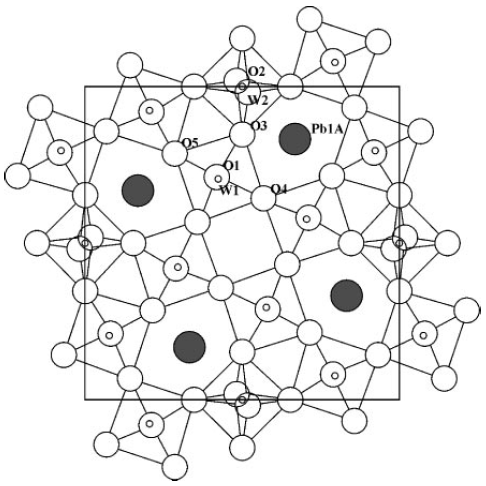
Figure 3352b. Average lead-TTB structure obtained by XRD technique and projected onto the ab plane. [12]
Important structural modifications from "ideal" TTB can occur because of the existence of tunnels. For instance, some big tunnels possibly provide the diffusion paths for the oxidation and can easily be hosts of elements. Figure 3352c (a) shows the structural model of Nb8W9O47 (orthorhombic, P21212, a = 1.224 nm; b = 3.658 nm; and c = 0.394 nm) in projection onto the ab-plane. The filled circles represent the pentagonal tunnels occupied by M-O (M = Nb or W) strings. Figure 3352c (b) shows a HRTEM image of Nb4W13O47 along [001]. The inset shows a highlighted representation of the structure: the positions of octahedrally coordinated cation sites are connected by lines, while the the pentagonal tunnels occupied by cations are indicated by open circles. The two distinct orientations of the TTB unit (white lines) and a unit cell (black rectangle) are outlined.
![The structural model of Nb8W9O47 in projection onto the ab-plane and (b) a HRTEM image of Nb4W13O47 along [001]](image1/3352b.jpg)
Figure 3352c. (a) The structural model of Nb8W9O47 in projection onto the
ab-plane and (b) a HRTEM image of Nb4W13O47 along [001]. [5]
Figure 3352d shows (a) a HRTEM image, (b) the HRTEM image with a simplified TTB-structural model superimposed and (c) the TTB-structural model of a niobium tungsten oxide (Nb4W13O47) of TTB-type after reacted by in situ TEM oxidation by an injected oxygen gas. The hexagonal and heptagonal tunnels appear with bright contrast at Scherzer defocus [6]. The most part of the oxidized Nb4W13O47 shows complicated and irregular framework of octahedra because of disordering by in situ oxidation. Some parts contain microdomains with regular arrangements of the TTB units that corresponds to a new intergrowth structure between ReO3 and TTB types.
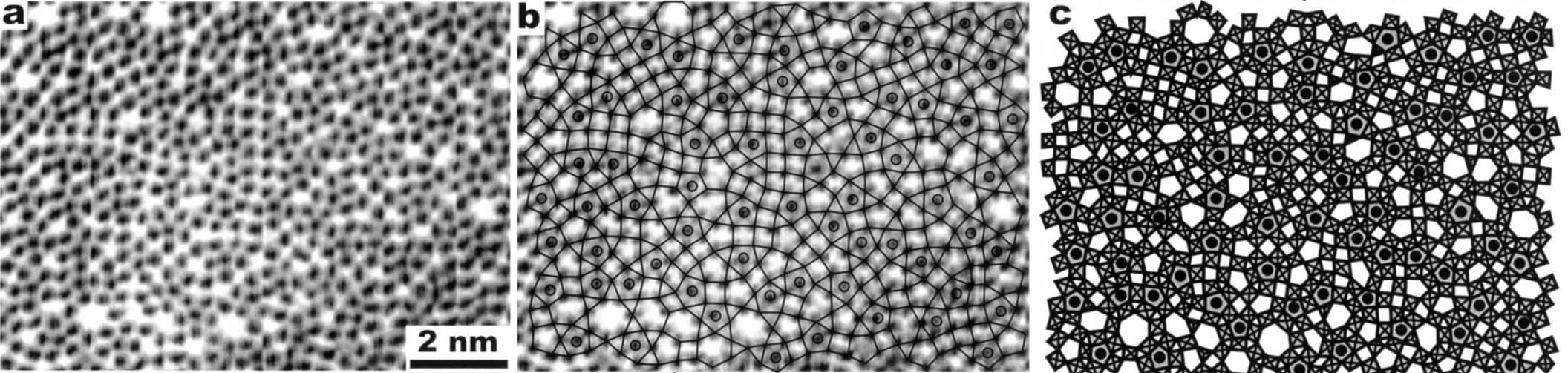
Figure 3352d. (a) HRTEM image, (b) HRTEM image with the simplified TTB-structural model
superimposed and (c) TTB-structural model of Nb4W13O47. [5]
Diffuse scattering in electron diffraction can be induced by disordering or less-ordering of some atomic arrangements. For instance, two niobium tungsten oxides (Nb7W10O47.5 and Nb4W13O49) consist often of less-ordered arrangements of filled tunnels, causing the diffuse scattering in electron diffraction. Figure 3352e shows the electron diffraction patterns (along [001]) of two crystallites of Nb7W10O47.5 presenting circular diffuse scattering in different degrees, which appears around the main bright reflections of the basic TTB substructure. In Nb4W13O49 as shown in Figure 3352f, the diffuse scattering pattern is cross-shaped due to the presence of long slabs of diamond-linked pentagonal columns. Overall, the origin of the diffuse scatterings is presumably the presence of short-range order in the PC (pentagonal columns) sublattice [7], or an intermediate state of order (or called transition state [8]) between a random arrangement and a long-range ordered structure. In addition, the weak spots indicate TTB superstructures.
![The electron diffraction patterns (along [001]) of two crystallites of Nb7W10O47.5](image1/2328a.gif)
Figure 3352e. The electron diffraction patterns (along [001]) of two crystallites of Nb7W10O47.5. Adapted from [9]
![The electron diffraction patterns (along [001]) of two crystallites of Nb4W13O49](image1/2328b.jpg)
Figure 3352f. The electron diffraction patterns (along [001]) of a Nb4W13O49 crystallite. Adapted from [9]
The analysis of Laue zones can provide detailed information regarding the samples. For instance, √2-TTB phase of PbxNb1.17W1.0O5.93+x (x > 0.15) in Figure 3352g presented asymmetric electron diffraction patterns and exhibited systematically weak odd-order (First order here) Laue layers lying half-way between the positions of the strong even-order (zero and second order here) layers. The even-order layers correspond to the basic 3.8 Å cTTB (c axis of TTB structure) repeat. Analyses of multiple samples indicate that these weak odd-order reflections were present in the same positions as the maxima in the even layers and at the midpoint of each edge of the basic TTB square.
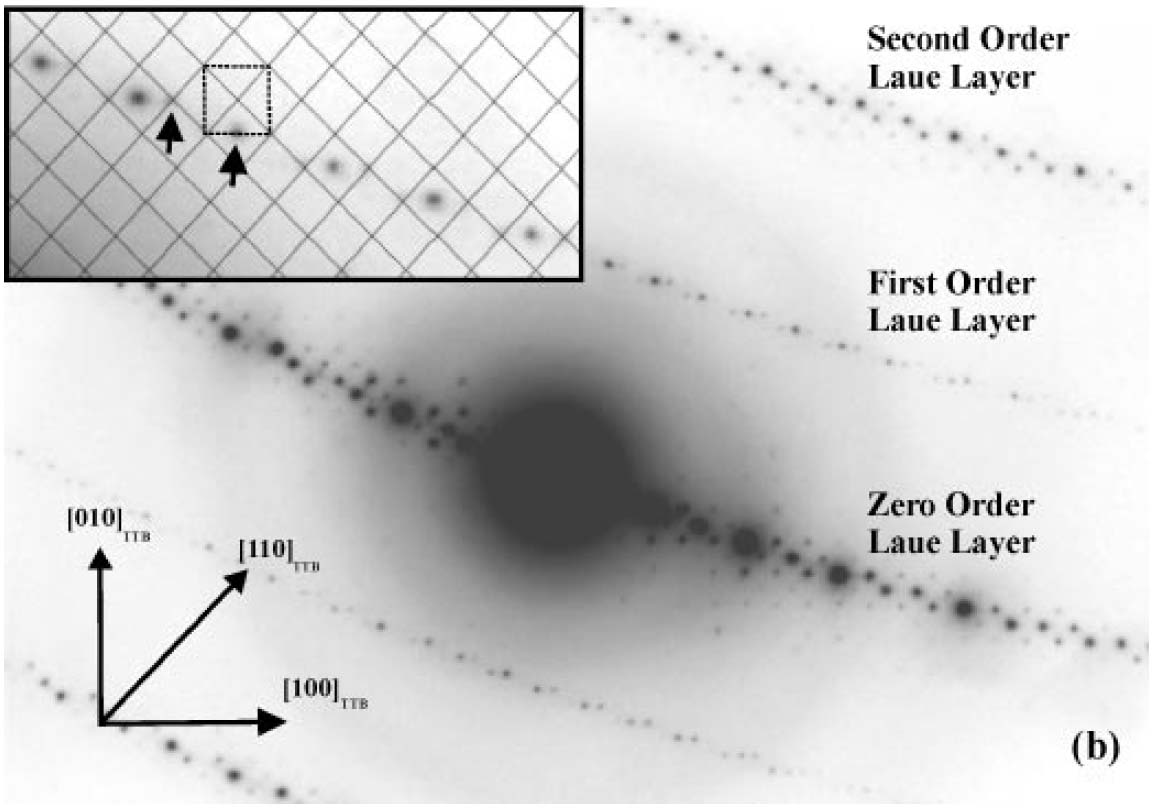
Figure 3352g. Asymmetric diffraction pattern of the √2-TTB phase of PbxNb1.17W1.0O5.93+x (x > 0.15). The inset enlargement of the first-order Laue layer shows that the black mesh defines the √2-TTB reciprocal lattice periodicity [11], and the dashed square illustrates the position of the basic TTB cell repeat with respect to the √2-TTB cell.
Adapted from [10]
[1] Magnéli, A., 1949. The crystal structure of tetragonal potassium tungsten bronze. Arkiv Kemi 24, 213 - 221.
[2] Hyde, B.G., O'Keeffe, M., 1973. Relations between the DO9 (ReO3) structure type and some bronze and tunnel structures. Acta Crystallogr. A 29, 243 - 248.
[3] Bovin, J.-O., Andersson, S., 1976. Chemical fourling on the unit cell level as a structure-building operation in the solid state. J. Solid State Chem. 18, 347 - 355.
[4] Labbé, P., 1992. Tungsten oxides, tungsten bronzes and tungsten bronzetype structures. In: Boulesteix, C. (Ed.). Diffuseless Phase Transitions and Related Structures in Oxides. Trans Tech, Clausthal, pp. 293 - 339.
[5] M.J. Sayagués, F. Krumeich, J.L. Hutchison, Solid-gas reactions of complex oxides inside an environmental high-resolution transmission electron microscope, Micron 32 (2001) 457 - 471.
[6] Sahle, W., 1983. Electron microscopy of tungsten oxides in the range WO3 - WO2.72. Phase relations, defect structures, structural transformations and electrical conductivity. Chem. Commun. Univ. Stockholm, vol. 4.
[7] Iijima, S. & Cowley, J. M. (1977). J. Phys. Colloq. 38, 135-144.
[8] Amelinckx, S. (1992). Materials Science and Technology, Vol. 2A, Characterisation of Materials, Part. I, pp. 1-146. Weinheim: Verlag Chemie.
[9] Frank Krumeich, Order and Disorder in Niobium Tungsten Oxides of the Tetragonal Tungsten Bronze Type, Acta Cryst. (1998). B54, 240-249.
[10] Sarah K. Haydon and David A. Jefferson, Quaternary Lead-Niobium-Tungsten Oxides Based on the Tetragonal Tungsten Bronze Structure, Journal of Solid State Chemistry 161, 135 - 151 (2001).
[11] S. K. Haydon, Ph.D. Thesis, University of Cambridge, 2000.
[12]
S. T. Triantafyllou, P. C. Christidis, and Ch. B. Lioutas, J. Solid State
Chem. 130, 176 - 183 (1997).
|


![The structural model of Nb8W9O47 in projection onto the ab-plane and (b) a HRTEM image of Nb4W13O47 along [001]](image1/3352b.jpg)

![The electron diffraction patterns (along [001]) of two crystallites of Nb7W10O47.5](image1/2328a.gif)
![The electron diffraction patterns (along [001]) of two crystallites of Nb4W13O49](image1/2328b.jpg)
