=================================================================================
In reality, complex cubic ABO3 perovskite structures can be formed. For instance, the B-site cations adopt a doubled perovskite unit cell, A(B'1/2B"1/2)O3-type 1:1 structure with a face centered arrangement of the two different (B' and B") cation positions. [1] For simplicity, A-site cation and oxygen are not shown in the complex cubic perovskite structure in Figure 3528, while the {111} B planes are highlighted in green and red. We can see every other {111} plane belongs to B' sublattice (in green) but the remaining {111} planes belong to B" sublattice (in red).
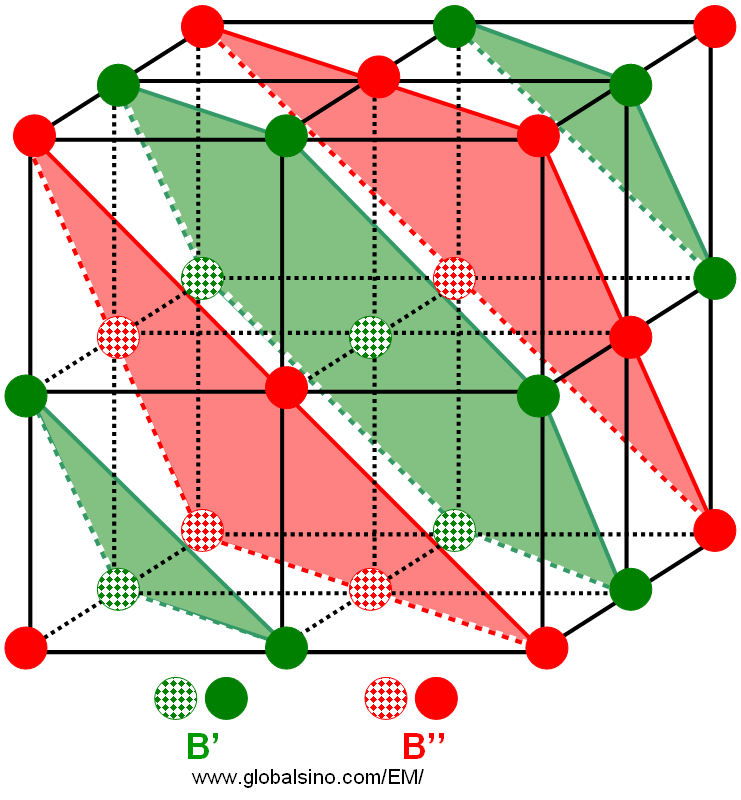
Figure 3530. Double perovskite crystalline structures.
As an example, lead magnesium niobate [Pb(Mgm/nNbn-m/n)O3, PMN] at room temperature, where Pb2+ sits on A-sites while Mg2+ and Nb5+ share the B-sites (namely, the octahedral center in the perovskite structures). The PMN crystals can be in the chemical form of ordered nanodomains. In this model, in some domains dispersed in the disordered PMN matrix only one of the two cation sublattices in 1:1 ordered PMN (B") is occupied by a single type of metal ion (e.g. Nb5+ in PMN), while in other domains the other cation sublattice (B') contains a random 2:1 distribution of Mg2+ and Nb5+ cations.
[1] G. A. Smolenskii and A. I. Agranovskaya, Soviet Physics - Technical Physics. 3, 1380 (1958).
|
