=================================================================================
EELS data on the occupancies of 3d and 4d states can clarify many fundamental problems related to the electronic properties of transition metal alloys. Table 4696a shows the 4d elements in periodic table.
Table 4696a. 4d elements in periodic table
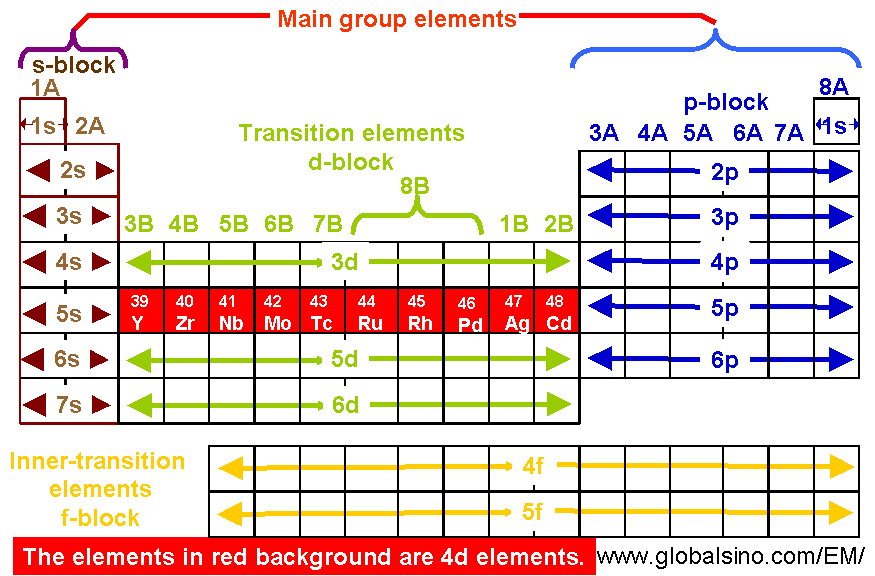
Table 4696b. Electron configurations, filling orbitals, and valence electrons of 4d elements
| Atomic Number |
Symbol |
Name |
Electron Configuration |
Filling Orbital |
Valence Electrons |
| 39 |
Y |
Yttrium |
1s2 2s2p6 3s2p6d10 4s2p6d1 5s2 |
4d1 |
4d15s2 |
| 40 |
Zr |
Zirconium |
1s2 2s2p6 3s2p6d10 4s2p6d2 5s2 |
4d2 |
4d25s2 |
| 41 |
Nb |
Niobium |
1s2 2s2p6 3s2p6d10 4s2p6d4 5s1 |
4d4 |
4d45s1 |
| 42 |
Mo |
Molybdenum |
1s2 2s2p6 3s2p6d10 4s2p6d5 5s1 |
4d5 |
4d55s1 |
| 43 |
Tc |
Technetium |
1s2 2s2p6 3s2p6d10 4s2p6d5 5s2 |
4d6 |
4d65s1 |
| 44 |
Ru |
Ruthenium |
1s2 2s2p6 3s2p6d10 4s2p6d7 5s1 |
4d7 |
4d75s1 |
| 45 |
Rh |
Rhodium |
1s2 2s2p6 3s2p6d10 4s2p6d8 5s1 |
4d8 |
4d85s1 |
| 46 |
Pd |
Palladium |
1s2 2s2p6 3s2p6d10 4s2p6d10 |
4d10 |
4d10 |
| 47 |
Ag |
Silver |
1s2 2s2p6 3s2p6d10 4s2p6d10 5s1 |
4d10 |
4d105s1 |
| 48 |
Cd |
Cadmium |
1s2 2s2p6 3s2p6d10 4s2p6d10 5s2 |
4d10 |
5s2 |
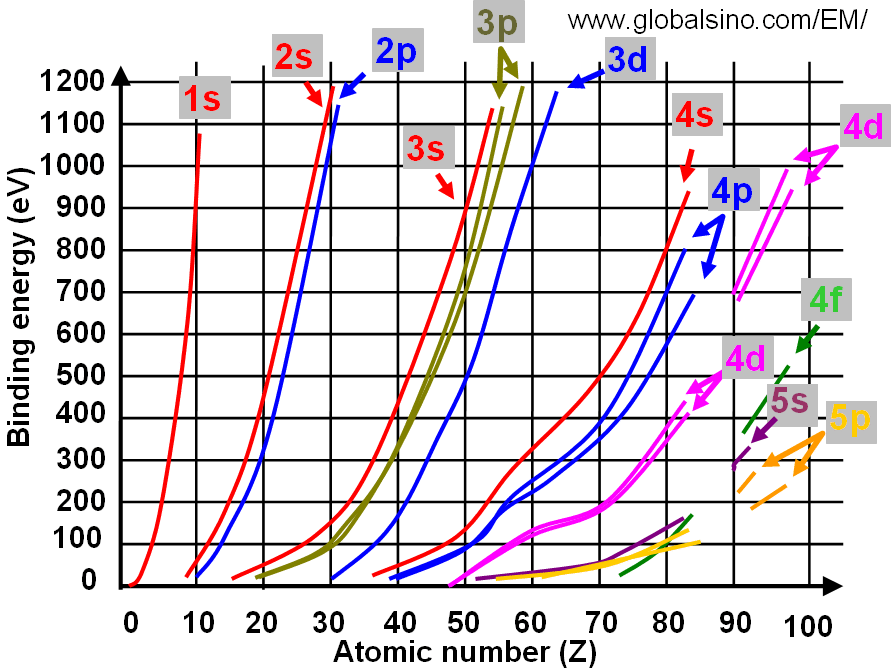
Figure 4696 shows the relation between electron binding energies and atomic number (Z).
Figure 4696. Relation between electron binding energies and atomic number (Z) (at low binding energies).
|