
Chapter/Index: Introduction | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Appendix
| EELS data on the occupancies of 3d and 4d states can clarify many fundamental problems related to the electronic properties of transition metal alloys. Table 4697a shows the 3d elements in periodic table Table 4697a. 3d elements in periodic table.
Table 4697b. Electron configurations, filling orbitals, and valence electrons of 3d elements.
Furthermore, for instance, Mn (manganese) orbital configuration, [Ar]4s23d5, allows for a range of possible valence states. The most prevalent configuration in nature are Mn2+, Mn3+, and Mn4+. Figure 4697a shows the relation between electron binding energies and atomic number (Z). 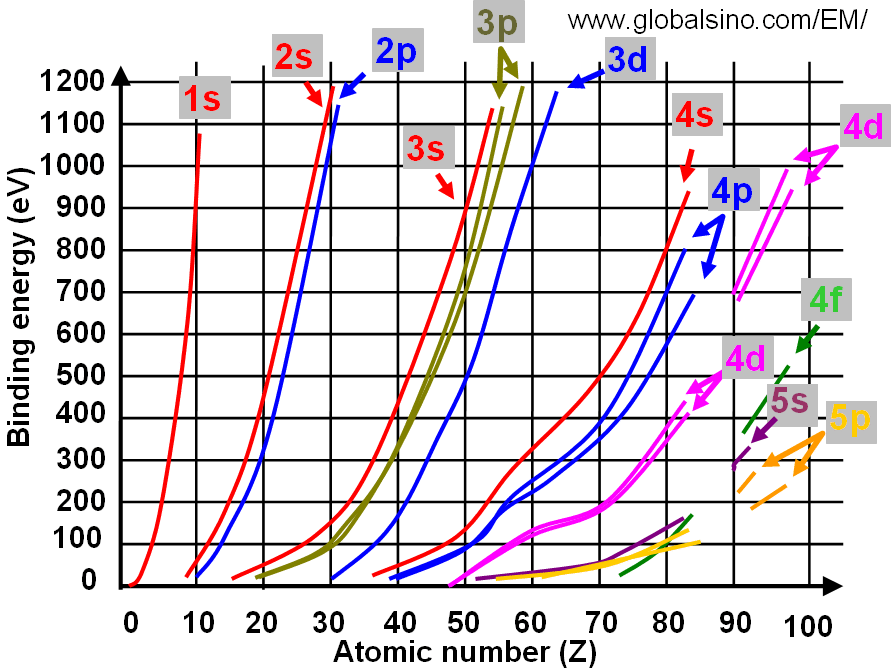 Figure 4697a. Relation between electron binding energies and atomic number (Z) (at low binding energies). The ionic radii decrease with increase in atomic number as shown in Table 4697c and Figure 4697b. Table 4697c. Ionic radii of 3d elements with different valence states.
Figure 4697b. Ionic radii of 3d elements.
|