=================================================================================
A crystal possesses a spontaneous polarization (Ps) if the centers of the positive and negative charges in the crystal structure do not coincide naturally. Spontaneous polarization can be quantified by the value of electrical dipole moments per unit volume in units of C-m/m3. Ferroelectric substances normally present separate regions called domains that have different spontaneous polarization directions. The number of distinct spontaneous polarization directions depends on its point group symmetry. Moreover, the rotation of polarization is accompanied by a change of crystal orientation and/or symmetry.
The spontaneous polarization in ferroelectrics has two or more orientational states and may be switched from one state to the other by an external electric field, or in some cases, by a mechanical stress. The ability to switch the polarization depends on the experimental conditions and the material properties (e.g. crystalline perfection and electrical conductivity). One classification method of ferroelectric substances is based on the number of directions allowed to spontaneous polarization:
a) Spontaneous polarization along
single axis.
a.1) Seignette salt.
a.2) Potassium di-hydrogen phosphate.
a.3) (NH4)2SO4.
a.4) (NH4)2BF4.
a.5) Colemanite.
a.6) Thiourea.
a.7) Glycine sulfate.
a.8) Glycine selenate.
b) Spontaneous polarization along
multiple axes.
b.1) Perovskite ferroelectric materials.
These materials are barium titanate, lead titanate (PbTiO3), lead zirconate titanate (Pb(Zr,Ti)O3), lead lanthanum zirconate titanate ((Pb,La)(Zr,Ti)O3), lead magnesium niobate (PMN), KNbO3, potassium sodium niobate (KxNa1-xNbO3), and potassium tantalate niobate (K(TaxNb1-x)O3).
b.2) Pyrochlore
ferroelectric materials (e.g. Cd2Nb2O7).
b.3) Niobate
ferroelectric materials.
b.4) Alums, such as CH3NH3Al(SO4)2•12H2O and (NH4)2Cd2(SO4)3.
c) Undefined.
c.1) Ilmenite ferroelectric materials such as LiNbO3 and LiTaO3.
The comparison between the pyroelectrics and ferroelectrics is:
i) Below the Curie temperature, both the pyroelectric and ferroelectric substances develop spontaneous polarizations.
ii) If the temperature is raised back above the Curie temperature, the polarization disappears again for both pyroelectric and ferroelectric substances.
iii) When a reversed electric field is applied, the spontaneous polarization reverses in the ferroelectric substances, but not in the pyroelectric substances.
Figure 2380 shows a typical polarization versus electric field loop of a ferroelectric material.
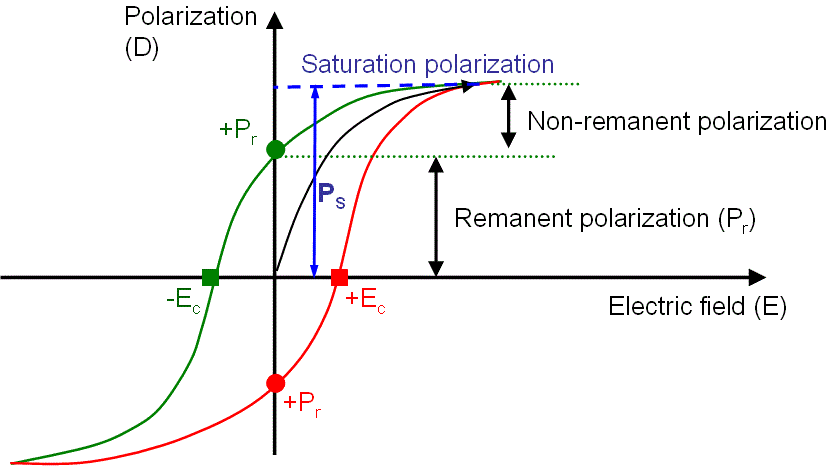
Figure 2380. A typical dielectric hysteresis loop (Ec: coercive field; Ps: spontaneous polarization; and Pr: remanent polarization).
|
