=================================================================================
The gap between the top of valence band and the bottom of conduction band in solids is also defined as energy gap Eg that is called band gap as well. That is, between the valence band and the conduction band the electrons find no energy levels that they can occupy. In insulators, the energy gap is very large and no vacant conduction band is available to the valence electrons. Otherwise, the energy gap is either zero due overlapping between valence and conduction bands for metals or is very small for semi-conductors so that conduction band is easily available to valence electrons.
Energy band is a continuous range of allowed and closely spaced energy levels for electrons in solids, e.g. crystalline materials. Individual atoms can have only certain quantized energy levels as shown on the left in Figure 3789. As atoms bond to form a solid, the wavefunctions of the individual electrons overlap with those of electrons confined to neighboring atoms, therefore, each energy level widens to accommodate the shifted levels of adjacent atoms. The energy bands can be though of as the collection of the individual energy levels of electrons surrounding each atom. Figure 3789 shows the difference of energies between an isolated Na atom and a Na solid. Bandgap is the range of energies between two allowed energy bands.
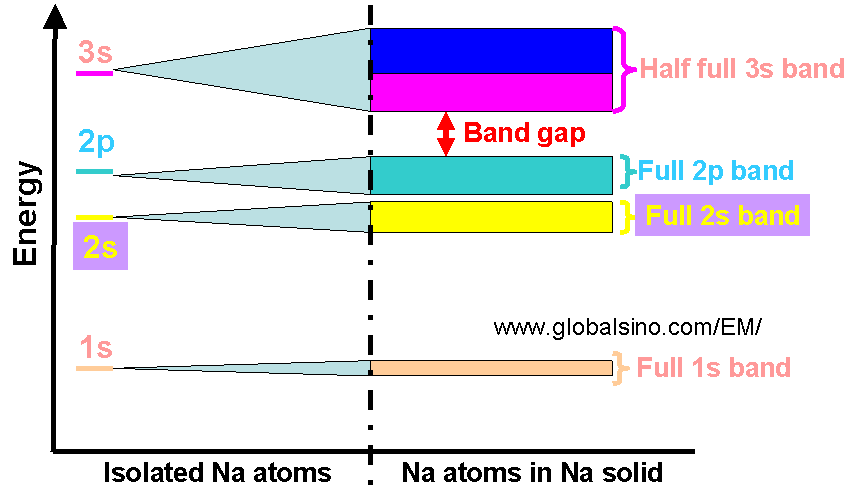
Figure 3789. Difference of energies between an isolated Na atom and a Na solid.
Furthermore, Table 3789 lists the electronic properties that are affected by structural disorders in amorphous and crystalline Si.
Table 3789. The electronic properties affected by structural disorders in amorphous and crystalline Si.
Electronic properties |
Structural disorders |
|
Bonding disorder |
Electronic states in band gap
|
Structural defects |
Electronical, metastable states |
Alternative bonding configurations |
|
