|
|
Iron (Fe)
- Practical Electron Microscopy and Database -
- An Online Book -
|
|
http://www.globalsino.com/EM/
|
|
This book (Practical Electron Microscopy and Database) is a reference for TEM and SEM students, operators, engineers, technicians, managers, and researchers.
|
=================================================================================
Fe is by far the most common 3d transition metal in minerals
Table 3395a. Properties of Fe metal.
Density (g-cm-3) |
7.87 |
Specific heat (cp, Jkg-1K-1)
|
0.46 |
Heat conductivity (kh,Wcm-1K-1)
|
0.75 |
Melting temperature (Tm, °C)
|
1537 |
Vaporization temperature (Tv, °C)
|
2735 |
|
274 |
Vaporization heat (Qv, J-g-1)
|
6365 |
Absorption (1-R) |
0.35 for 1 µm light |
K α X-ray for XRD measurements |
Wavelength (λ) = 0.194 nm |
As listed in Table 3395b, substances with large bonding energies usually have high melting temperatures.
Table 3395b. Bonding energies and melting temperatures of metallic substances.
Bonding type |
Substance |
Bonding energy |
Melting point (°C) |
| kJ/mol |
kcal/mol |
eV/Atom, Ion, or Molecule |
Metallic
|
Typical value |
50-1000 |
|
|
|
| Hg |
68 |
16 |
0.7 |
-39 |
| Al |
324 |
77 |
3.4 |
660 |
| Fe |
406 |
97 |
4.2 |
1538 |
| W |
849 |
203 |
8.8 |
3410 |
Figure 3395 shows the solid solubility of some impurities (including Fe) in silicon.
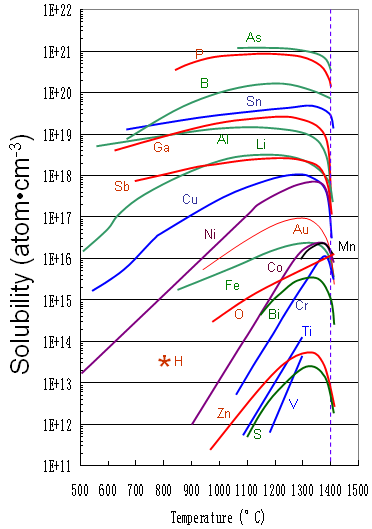
Figure 3395. Solid solubility of impurities in silicon.
For TEMs with thermionic electron sources, the MDM (minimum detectable mass) of EDS measurements is in the range of 10-19 to 10-20 g, equivalent to ~100 - ~1000 atoms thick of iron (Fe). For TEMs with FEGs (field emission guns), it is possible to detect a few atoms thick with EDS technique.
|
=================================================================================
The book author (Yougui Liao) welcomes your comments, suggestions, and corrections, please click here for submission. If you let book author know once you have cited this book, the brief information of your publication will appear on the “Times Cited” page.
|
|