=================================================================================
An interband transition is an electronic transition between different bands (inter- is the Latin word for ‘between’). Different from intraband transitions, interband transitions are avoidable if you work at frequencies away from the bandgap energy.
Interband transitions are excited from occupied valence bands and shallow core levels to the unoccupied conduction bands. The electronic and optical interband transitions are categorized by direct and indirect transitions because the conduction band bottom and the valance band top contain more than one extrema. The electronic and optical band gaps can be calculated by the predominant mechanism of band to band transitions and are normally less than several volts. In the allowed direct transition, the electrons in the valence band transit vertically to the conduction band under the excitation of incident electrons or photons. The absorption coefficient as a function of photon energy in the allowed direct transition can be given by [1],
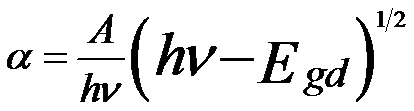 ------------------------------ [3952a] ------------------------------ [3952a]
where,
A -- A constant
v -- The frequency
h -- The Planck constant
Egd -- The allowed direct energy gap.
The interband transitions correspond to the absorption peaks in the imaginary part of dielectric function ε2. On the other hand, it is strongly modulated by ε21 + ε22 in energy loss function, which dumps the peak strength of interband transitions.
In the indirect transition, the electrons transit from the valence band top to the conduction band bottom with participation of photons and appropriate phonons. In this case, the absorption coefficient can be given by [2],
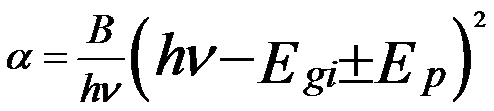 ------------------------------ [3952b] ------------------------------ [3952b]
where,
B -- A constant
Egi -- The indirect energy gap
Ep -- The energy of the absorbed (+) or emitted (-) phonons.
Interband transitions are an effect of the lattice. They shift the plasmon energy and can be included via the Drude–Lorentz theory. [3 - 5] The energy region of the EEL spectrum (EELS) up to the energy loss of ∼50 eV is dominated by the collective excitations of valence electrons (plasmon) and by interband transitions. In order to include interband transitions, we need to have a good understanding of the electronic structure. The EELS of the low-energy loss region less than 50 eV is particularly called valence electron energy loss spectroscopy (VEELS).
Figure 3952a shows the EEL spectra of plasmon region (zero-peak is not shown) for crystalline and amorphous diamond. The loss function (Im[-l/ε]) can then be obtained by removing contribution from multiple scattering using the Fourier-log deconvolution method.
Figure 3952a. EEL spectra of plasmon region for (a) crystalline and (b) amorphous diamond.
An optical absorption method can directly provide the imaginary part of the dielectric function, ε2, associated with a single electron excitation of an interband transition, while EELS cannot directly give ε2. Based on the obtained loss function, the real part (ε1), and the imaginary part (ε2) of the dielectric function for the crystalline and the amorphous diamond are extracted using Kramers-Kronig analysis as shown in Figure 3952b. Table 3952 lists the onset energy (indicating the band gap energy) and the peaks in the imaginary spectrum obtained from Figure 3952b and the original interband transitions.
Figure 3952b. Real part (ε1), and the imaginary part (ε2) of the dielectric function for (a) crystalline and (b) amorphous diamond.
Table 3952. Onset energies (band gap energies), peaks in the imaginary spectrum in Figure 3952b and the original interband transitions.
| |
Onset energy (band gap energy) |
Peaks in imaginary spectrum |
Interband transition |
Crystal diamond |
5.5 eV |
8.2 |
Γ point |
| 12.7 |
X and L points |
Amorphous diamond |
4.0 eV |
7.2 eV |
Γ point |
Note if the interband transitions are in the energy range less than ~2 eV, these spectral features are very close to the intense zero-loss peak from elastic scattering, so that it is difficult to resolve them.
Page3375 lists the behaviors and properties of various inelastic electron scatterings in electron interaction with materials, including inter- and intra-band transitions, inner shell ionization, phonon excitation and plasmon excitation.
[1] Tauc JC (1972) Optical properties of solids. North-Holland,
Amsterdam.
[2] El-Korashy A, El-Fadl AA (1999) Phys B 271:205.
[3] R.F. Egerton, Electron Energy Loss Spectroscopy in the Electron
Microscope, Plenum Press, New York, 1996.
[4] P. Schattschneider, B. Jouffrey, in: L. Reimer (Ed.), Energy-filtering
Transmission Electron Microscopy, Springer, 1995, p. 151.
[5] H. Raether, Excitation of Plasmons and Interband Transitions by
Electrons, Springer-Verlag, Berlin, 1980.
|