=================================================================================
An atom is ionized when an inner shell electron is removed by high-energy-electron radiation. In this step, vacancies in inner shell are formed. To return the excited atom to its ground state, the electron from an outer, higher energy shell fills the vacant inner shell, and then the atom releases an amount of energy equal to the potential energy difference between the two shells. This excess energy, which is unique for every atomic transition, will be emitted by the excited atom either as an X-ray photon (for EDS measurement: Energy Dispersive X-ray Spectrum) or will be self-absorbed and emitted as an Auger electron (for AES measurement: Auger Electron Spectrum). Auger electrons and X-rays are the only possible outcomes, and the sum of the two probabilities is unity.
Table 4660. Comparison between EDS and AES.
|
EDS |
AES |
Naming |
Named by the initial core-hole state, the emission line, and the line strength (1 is the strongest), e.g. 3d5/2 → 2p3/2 is Lα1 [see page4478] |
Named by initial ionization, filling shell, shell of ejected electron, e. g. KL2L3 [see page4478] |
|
Dominates over Auger processes for deeper core relaxation |
Weaker information for deeper core relaxation |
Characteristic of
excited atoms
|
Yes |
Yes |
|
The relatively large mean free paths
of X-rays, particularly those of high-energy photons, allows signals to be
collected from a large volume of the sample probed by the electron
beam. |
The relatively short
mean free path of the Auger electrons in bulk solids (on the order of few
nanometers) means that only those produced near the surface layer can escape
to the free space to be collected. This restricts the application of Auger
spectroscopy to surface study. |
|
The typical energy resolution
for EDS is of on the order of 150 eV. This is suffcient in most cases
for resolving peaks of different elements, but is inadequate for detecting chemical shifts of the atoms which are of the order of a few electronvolts. EDS
is thus mainly used for composition analysis. |
|
Yield at low accelerating voltages of incident electrons
|
Very few x-rays are emitted |
Auger electron emission dominates |
Figure 4660a shows both generation processes of x-rays and Auger electron.
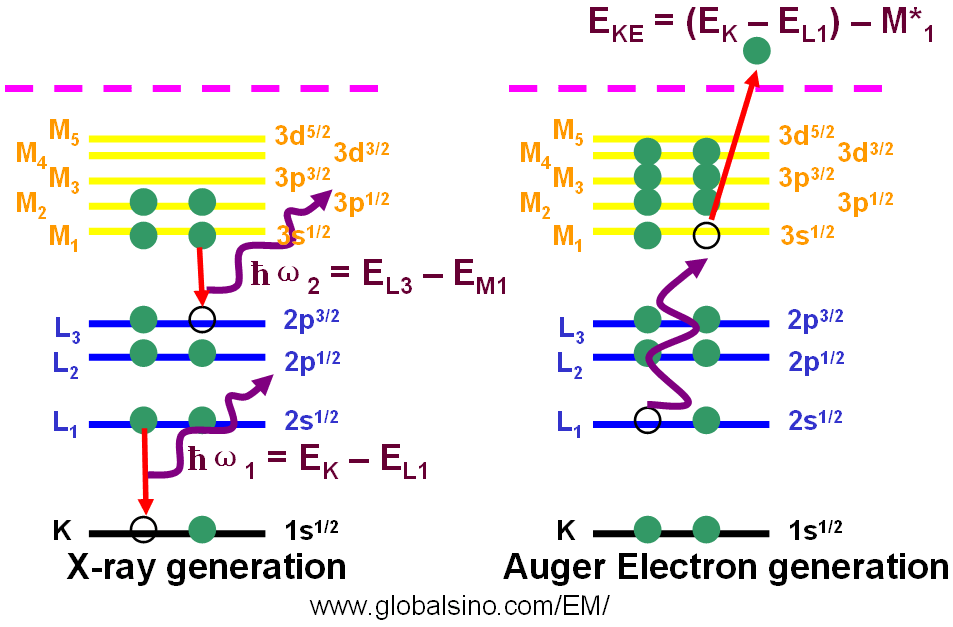
Figure 4660a. Generation processes of X-rays and Auger electron.
As an example, Figure 4660b shows the X-ray and Auger electron yields per K vacancy as a function of atomic number. For low-Z material Auger emission is dominant, while for high-Z material X-ray emission dominates. The two emission probabilities are approximately equal at Z ≈ 30 for K shell ionization.
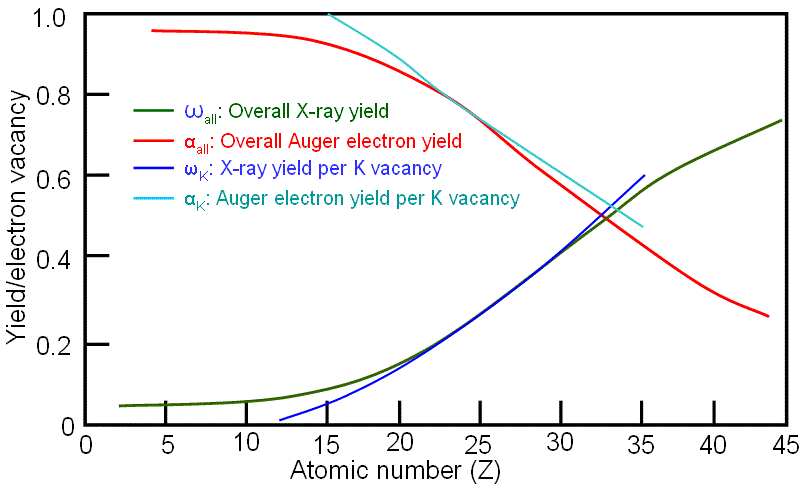
Figure 4660b. X-ray and Auger electron yields per K vacancy as a function of atomic number.
Figure 4660c shows the escape depths of various species generated by high-energy electrons penetrating into a solid. Auger electrons and "soft" X-rays are emitted in depth of <1 nm and in diameter of beam size, secondary electrons are emitted in depth of < 10 nm and in diameter of beam size, and the emission depths and diameters of backscattered electrons and "hard" X-rays depend on both the beam energy and beam size.
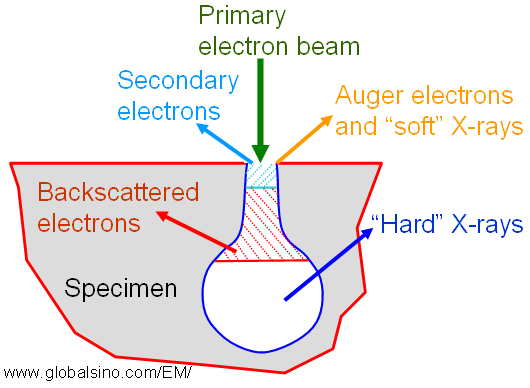
Figure 4660c. Escape depths of various species generated by high-energy electrons penetrating into a solid.
In comparison between the emissions of Auger electrons and “soft” X-rays, for electron irradiation of high atomic-number (Z) elements, Auger electron emission predominates, while for irradiation of low Z elements, “soft” X-rays predominate.
|