=================================================================================
A specific terminology, called shell, can be used to identify the different characteristic X-rays. We can think an electron shell as an orbit followed by electrons around an atom's nucleus. According to the simple Bohr theory of atomic structure, the electrons are circling the nucleus in specific shells. In this case, the electrons stay in their shells rather than spiral into the nucleus because of the constraints imposed by quantum theory. As shown in Figure 4678, for historical reasons, the innermost electron shell is called the K shell and the next innermost is the L shell, the next the M, and so on.
 |
| Figure 4678a.
Charles Glover Barkla
was born on 7 June 1877, in Widnes, United Kingdom, and died on 23 October 1944, Edinburgh, Scotland.
His affiliation was at the time of the award: Edinburgh University, Edinburgh, United Kingdom.
He received his Nobel Prize one year later, in 1918 with prize motivation of "for his discovery of the characteristic Röntgen radiation of the elements"
in the field of atomic physics. |
Why do the electron shells (orbits in the Bohr model) begin being named with letters K, L, M, N, and not with A, B, C? The K, L, M-shell labels were not proposed by Bohr as part of his original 1913 quantum model of the atom. The K, L, M, etc., terminology [1] was first introduced by Charles Barkla, an early X-ray spectroscopist, who experimentally studied the X-rays that are emitted by atoms when they are hit with high energy electrons. He noticed that atoms appeared to emit two types of X-rays. The two types of X-rays differed in energy and Barkla originally called the higher energy X-ray type A and the lower energy X-ray type B. He later renamed these two types K and L since he realized that the highest energy X-rays produced in his experiments might not be the highest energy X-ray possible. He wanted to make certain that there was room to add more discoveries without ending up with an alphabetical list of X-rays whose energies were mixed up.
As it turns out, the K type X-ray is the highest energy X-ray an atom can emit. It is produced when an electron in the innermost shell is knocked free and then recaptured. This innermost shell is now called the K-shell, after the label used for the X-ray. Barkla won the 1917 Nobel Prize for Physics for this work.
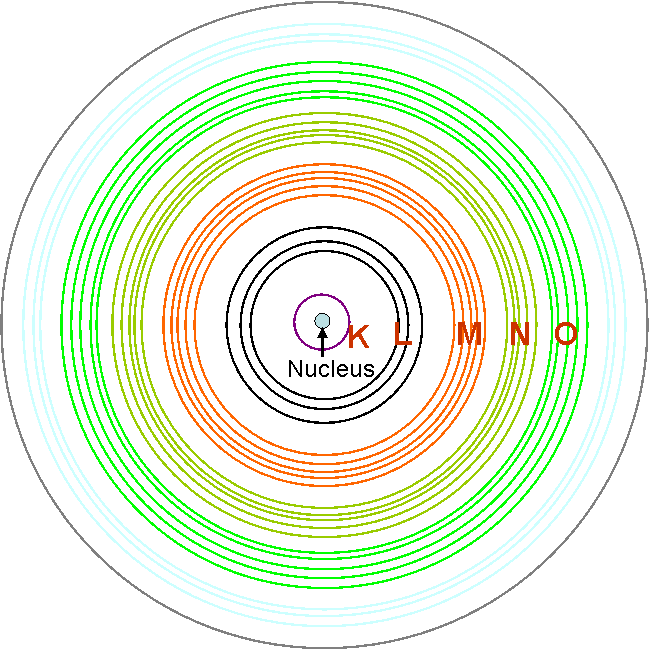
Figure 4678. Schematic of electron shells.
The number of electrons in each shell of all chemical elements in periodic table is listed in Table 4678.
Table 4678. Electron number in each shell of all chemical elements in periodic table.
Atomic number (Z) |
Element |
No. of electrons/shell |
Group |
|
|
Shell: K, L, M, N, O |
|
1 |
Hydrogen |
1 |
1 |
2 |
Helium |
2 |
18 |
3 |
Lithium |
2, 1 |
1 |
4 |
Beryllium |
2, 2 |
2 |
5 |
Boron |
2, 3 |
13 |
6 |
Carbon |
2, 4 |
14 |
7 |
Nitrogen |
2, 5 |
15 |
8 |
Oxygen |
2, 6 |
16 |
9 |
Fluorine |
2, 7 |
17 |
10 |
Neon |
2, 8 |
18 |
11 |
Sodium |
2, 8, 1 |
1 |
12 |
Magnesium |
2, 8, 2 |
2 |
13 |
Aluminium |
2, 8, 3 |
13 |
14 |
Silicon |
2, 8, 4 |
14 |
15 |
Phosphorus |
2, 8, 5 |
15 |
16 |
Sulfur |
2, 8, 6 |
16 |
17 |
Chlorine |
2, 8, 7 |
17 |
18 |
Argon |
2, 8, 8 |
18 |
19 |
Potassium |
2, 8, 8, 1 |
1 |
20 |
Calcium |
2, 8, 8, 2 |
2 |
21 |
Scandium |
2, 8, 9, 2 |
3 |
22 |
Titanium |
2, 8, 10, 2 |
4 |
23 |
Vanadium |
2, 8, 11, 2 |
5 |
24 |
Chromium |
2, 8, 13, 1 |
6 |
25 |
Manganese |
2, 8, 13, 2 |
7 |
26 |
Iron |
2, 8, 14, 2 |
8 |
27 |
Cobalt |
2, 8, 15, 2 |
9 |
28 |
Nickel |
2, 8, 16, 2 |
10 |
29 |
Copper |
2, 8, 18, 1 |
11 |
30 |
Zinc |
2, 8, 18, 2 |
12 |
31 |
Gallium |
2, 8, 18, 3 |
13 |
32 |
Germanium |
2, 8, 18, 4 |
14 |
33 |
Arsenic |
2, 8, 18, 5 |
15 |
34 |
Selenium |
2, 8, 18, 6 |
16 |
35 |
Bromine |
2, 8, 18, 7 |
17 |
36 |
Krypton |
2, 8, 18, 8 |
18 |
37 |
Rubidium |
2, 8, 18, 8, 1 |
1 |
38 |
Strontium |
2, 8, 18, 8, 2 |
2 |
39 |
Yttrium |
2, 8, 18, 9, 2 |
3 |
40 |
Zirconium |
2, 8, 18, 10, 2 |
4 |
41 |
Niobium |
2, 8, 18, 12, 1 |
5 |
42 |
Molybdenum |
2, 8, 18, 13, 1 |
6 |
43 |
Technetium |
2, 8, 18, 13, 2 |
7 |
44 |
Ruthenium |
2, 8, 18, 15, 1 |
8 |
45 |
Rhodium |
2, 8, 18, 16, 1 |
9 |
46 |
Palladium |
2, 8, 18, 18 |
10 |
47 |
Silver |
2, 8, 18, 18, 1 |
11 |
48 |
Cadmium |
2, 8, 18, 18, 2 |
12 |
49 |
Indium |
2, 8, 18, 18, 3 |
13 |
50 |
Tin |
2, 8, 18, 18, 4 |
14 |
51 |
Antimony |
2, 8, 18, 18, 5 |
15 |
52 |
Tellurium |
2, 8, 18, 18, 6 |
16 |
53 |
Iodine |
2, 8, 18, 18, 7 |
17 |
54 |
Xenon |
2, 8, 18, 18, 8 |
18 |
55 |
Caesium |
2, 8, 18, 18, 8, 1 |
1 |
56 |
Barium |
2, 8, 18, 18, 8, 2 |
2 |
57 |
Lanthanum |
2, 8, 18, 18, 9, 2 |
|
58 |
Cerium |
2, 8, 18, 19, 9, 2 |
|
59 |
Praseodymium |
2, 8, 18, 21, 8, 2 |
|
60 |
Neodymium |
2, 8, 18, 22, 8, 2 |
|
61 |
Promethium |
2, 8, 18, 23, 8, 2 |
|
62 |
Samarium |
2, 8, 18, 24, 8, 2 |
|
63 |
Europium |
2, 8, 18, 25, 8, 2 |
|
64 |
Gadolinium |
2, 8, 18, 25, 9, 2 |
|
65 |
Terbium |
2, 8, 18, 27, 8, 2 |
|
66 |
Dysprosium |
2, 8, 18, 28, 8, 2 |
|
67 |
Holmium |
2, 8, 18, 29, 8, 2 |
|
68 |
Erbium |
2, 8, 18, 30, 8, 2 |
|
69 |
Thulium |
2, 8, 18, 31, 8, 2 |
|
70 |
Ytterbium |
2, 8, 18, 32, 8, 2 |
|
71 |
Lutetium |
2, 8, 18, 32, 9, 2 |
3 |
72 |
Hafnium |
2, 8, 18, 32, 10, 2 |
4 |
73 |
Tantalum |
2, 8, 18, 32, 11, 2 |
5 |
74 |
Tungsten |
2, 8, 18, 32, 12, 2 |
6 |
75 |
Rhenium |
2, 8, 18, 32, 13, 2 |
7 |
76 |
Osmium |
2, 8, 18, 32, 14, 2 |
8 |
77 |
Iridium |
2, 8, 18, 32, 15, 2 |
9 |
78 |
Platinum |
2, 8, 18, 32, 17, 1 |
10 |
79 |
Gold |
2, 8, 18, 32, 18, 1 |
11 |
80 |
Mercury |
2, 8, 18, 32, 18, 2 |
12 |
81 |
Thallium |
2, 8, 18, 32, 18, 3 |
13 |
82 |
Lead |
2, 8, 18, 32, 18, 4 |
14 |
83 |
Bismuth |
2, 8, 18, 32, 18, 5 |
15 |
84 |
Polonium |
2, 8, 18, 32, 18, 6 |
16 |
85 |
Astatine |
2, 8, 18, 32, 18, 7 |
17 |
86 |
Radon |
2, 8, 18, 32, 18, 8 |
18 |
87 |
Francium |
2, 8, 18, 32, 18, 8, 1 |
1 |
88 |
Radium |
2, 8, 18, 32, 18, 8, 2 |
2 |
89 |
Actinium |
2, 8, 18, 32, 18, 9, 2 |
|
90 |
Thorium |
2, 8, 18, 32, 18, 10, 2 |
|
91 |
Protactinium |
2, 8, 18, 32, 20, 9, 2 |
|
92 |
Uranium |
2, 8, 18, 32, 21, 9, 2 |
|
93 |
Neptunium |
2, 8, 18, 32, 22, 9, 2 |
|
94 |
Plutonium |
2, 8, 18, 32, 24, 8, 2 |
|
95 |
Americium |
2, 8, 18, 32, 25, 8, 2 |
|
96 |
Curium |
2, 8, 18, 32, 25, 9, 2 |
|
97 |
Berkelium |
2, 8, 18, 32, 27, 8, 2 |
|
98 |
Californium |
2, 8, 18, 32, 28, 8, 2 |
|
99 |
Einsteinium |
2, 8, 18, 32, 29, 8, 2 |
|
100 |
Fermium |
2, 8, 18, 32, 30, 8, 2 |
|
101 |
Mendelevium |
2, 8, 18, 32, 31, 8, 2 |
|
102 |
Nobelium |
2, 8, 18, 32, 32, 8, 2 |
|
103 |
Lawrencium |
2, 8, 18, 32, 32, 8, 3 (?) |
3 |
104 |
Rutherfordium |
2, 8, 18, 32, 32, 10, 2 (?) |
4 |
105 |
Dubnium |
2, 8, 18, 32, 32, 11, 2 (?) |
5 |
106 |
Seaborgium |
2, 8, 18, 32, 32, 12, 2 (?) |
6 |
107 |
Bohrium |
2, 8, 18, 32, 32, 13, 2 (?) |
7 |
108 |
Hassium |
2, 8, 18, 32, 32, 14, 2 (?) |
8 |
109 |
Meitnerium |
2, 8, 18, 32, 32, 15, 2 (?) |
9 |
110 |
Darmstadtium |
2, 8, 18, 32, 32, 17, 1 (?) |
10 |
111 |
Roentgenium |
2, 8, 18, 32, 32, 17, 2 (?) |
11 |
112 |
Copernicium |
2, 8, 18, 32, 32, 18, 2 (?) |
12 |
113 |
Ununtrium |
2, 8, 18, 32, 32, 18, 3 (?) |
13 |
114 |
Ununquadium |
2, 8, 18, 32, 32, 18, 4 (?) |
14 |
115 |
Ununpentium |
2, 8, 18, 32, 32, 18, 5 (?) |
15 |
116 |
Ununhexium |
2, 8, 18, 32, 32, 18, 6 (?) |
16 |
117 |
Ununseptium |
2, 8, 18, 32, 32, 18, 7 (?) |
17 |
118 |
Ununoctium |
2, 8, 18, 32, 32, 18, 8 (?) |
18 |
[1] C. G. Barkla, “The Spectra of the Fluorescent Röntgen Radiations,” Phil. Mag, 1911, 22, 396-412. See especially the footnote, p. 406. |

