|
This book (Practical Electron Microscopy and Database) is a reference for TEM and SEM students, operators, engineers, technicians, managers, and researchers.
|
=================================================================================
The dew point is the temperature to which a given parcel of humid air must be cooled, at constant barometric pressure, for water vapor to condense into water. The condensed water is called dew. The dew point is a saturation temperature.
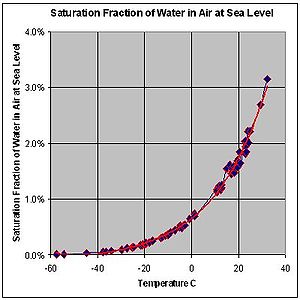 |
| This graph shows the maximum percentage (by mass) of water vapor that can exist in air at sea level across a range of temperatures. The behavior of water vapor does not depend on the presence of other gases in air. The formation of dew would occur at the dew point even if the only gas present were water vapor. |
The dew point is associated with relative humidity. A high relative humidity indicates that the dew point is closer to the current air temperature. Relative humidity of 100% indicates the dew point is equal to the current temperature and the air is maximally saturated with water. When the dew point remains constant and temperature increases, relative humidity will decrease.
The dew point is an important statistic for general aviation pilots, as it is used to calculate the likelihood of carburetor icing and fog, and to estimate the height of the cloud base.
This graph shows the maximum percentage (by mass) of water vapor that can exist in air at sea level across a range of temperatures. The behavior of water vapor does not depend on the presence of other gases in air. The formation of dew would occur at the dew point even if the only gas present were water vapor.
At a given temperature but independent of barometric pressure, the dew point is a consequence of the absolute humidity, the mass of water per unit volume of air. If the temperature rises without changing the absolute humidity, the dew point will rise and the relative humidity will lower accordingly. Reducing the absolute humidity will bring the dew point back down to its initial value. In the same way, increasing the absolute humidity after a temperature drop brings the dew point back down to its initial level. For this reason, the same relative humidity on a day when it's 80°F, and on a day when it's 100°F will imply that a higher fraction of the air on the hotter day consists of water vapor than on the cooler day, i.e., the dew point is higher.
At a given barometric pressure but independent of temperature, the dew point indicates the mole fraction of water vapor in the air, or, put differently, determines the specific humidity of the air. If the pressure rises without changing this mole fraction, the dew point will rise accordingly; Reducing the mole fraction, i.e., making the air less humid, would bring the dew point back down to its initial value. In the same way, increasing the mole fraction after a pressure drop brings the relative humidity back up to its initial level. Considering New York (33 ft elevation) and Denver (5,130 ft elevation), for example, this means that if the dew point and temperature in both cities are the same, then the mass of water vapor per cubic meter of air will be the same, but the mole fraction of water vapor in the air will be greater in Denver. |
