=================================================================================
Zirconium oxide (ZrO2) is a refractory material and is also known as zirconia or baddeleyite that is a rare zirconium oxide mineral. It has three solid phases (monoclinic, tetragonal and cubic crystals) as shown in Figure 2070a. It changes from monoclinic to tetragonal at 1400 K and to cubic at 2641 K as shown in Figure 2070b. The cubic phase has a fluorite crystal structure. The monoclinic-to-tetragonal change is accompanied by a large volume reduction of ~7.5%, resulting in significant stresses. Figure 2070a indicates that dense parts may be obtained by sintering of the cubic or tetragonal phase only.
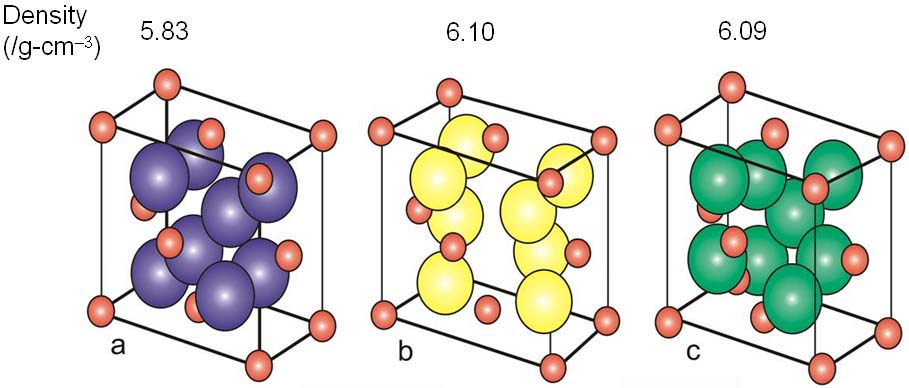
Figure 2070a. Crystal structure of monoclic (a), tetragonal (b) and cubic zirconia(c). Red spheres represent O atoms, while the other colors represent Zr atoms. Adapted from [1]
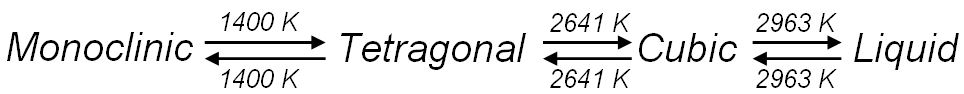
Figure 2070b. Phase transformation of zirconium oxide with increasing temperature.
Zr4+ in cubic ZrO2 has seven-fold coordination (Zr4+ is surrounded by seven oxygens), i.e., there are two types of O2- ions: O(l) with coordination number 3 and O(2) with coordination number 4. The distances between Zr and O are:
d(Zr-O(1)) = 0.209 nm
d(Zr-O(2)) = 0.221 nm
And, the average Zr-O distance in cubic ZrO2 is 0.216 nm.
However, in the tetragonal phase and
cubic phase the Zr4+ ion has eight-fold coordination.
Table 2070. Properties of ZrO2.
| |
|
|
Tetragonal modified-ZrO2 |
Rhombohedral ZrO2 |
|
Lattice parameters (Å) |
a = 5.1454,
b = 5.2075,
c = 5.3107,
α = 99.23° |
a = 5.094, c = 5.27 |
3 mol% Y2O3-ZrO2: a = 3.605 Å and c = 5.179 Å.
ZrO2: a = 3.598 Å and c = 5.165 Å |
|
5.065 |
Unit cell volume |
142.36 Å3 |
|
|
|
|
Strukturbericht
symbol |
C43 |
C4 |
|
|
Cl |
Pearson symbol |
mP12 |
tP6 |
|
|
cF12 |
Space group |
P21/c |
P42/mnm |
P42/nmc |
|
Fm-3m |
Coordination number |
7 |
|
|
|
|
Structure type |
Baddeleyite type |
Rutile type |
|
|
Fluorite type |
Polymorphism |
Monoclinic-tetragonal: 1273-1473; Tetragonal-cubic: 2643; Cubic-liquid: 2953 |
Temperature of stable structure (°C) |
<1100 |
|
1100 < t < 2100 |
|
> 2100 |
Static dielectric constant (K) |
|
|
|
|
25 |
|
|
|
|
|
1.5 |
Band gap energy (eV) |
|
|
|
|
5.8 |
Molecular Weight (g/mol) |
|
|
|
|
123.11 |
Color |
|
|
|
|
white |
Density (g/cm3) |
5.83 |
5.68 - 6.05 |
|
|
5.80-6.09 |
Melting Point (°C) |
2677-2710 |
2680 |
|
2677-2710 |
Thermal Conductivity (W/m•°C) |
1.5 at 25 °C, 1.675 at 100 °C, 2 at 1000 °C, 2.094 at 1300 °C |
Maximum useful temperature (°C) |
2400 |
Coefficient
of linear
thermal expansion (10-6/°C) |
7 (parallel to a-axis), 2 (parallel to b-axis), 13 (parallel to c-axis) |
9 (parallel to a-axis), 12 (parallel to c-axis) |
|
7.5-13 (depending on stabilizer type and amount) |
Specific heat
capacity
(cp,Jkg−1K−1) |
711 |
|
|
|
400 |
Heat of formation (kJ mol−1) |
1096.73 |
Boiling point (K) |
4548 |
Electrical Resistivity (μΩ•cm) |
|
7.7×107 |
|
|
1 x 1012 |
Refractive index |
2.17 to 2.20 |
Birefringence |
None |
Mohs hardness |
6.5 |
Vickers Hardness (GPa) |
|
12.2 |
|
|
16 |
Young’s Modulus (GPa) |
|
201 |
|
|
207 |
|
|
1074 |
|
|
900-1250 |
Compressive strength (MPa) |
|
7500 |
|
|
|
Tensile strength (MPa) |
|
420 |
|
|
|
Fracture toughness (MPa m1/2) |
|
6-15 |
|
|
|
Effective electron masses (me/m0)
|
|
|
|
Γ-X: 2.01 & 0.58; Γ-L: 0.78 & 0.78; Γ-K: 1.25 & 0.64 |
|
Effective hole masses (mh/m0) |
|
|
|
X-Γ: 0.32; X-U: 2.64; X-W: 3.05 |
|
Spectral emittance |
0.2 (Visible wavelengths) |
Dispersion |
0.058 - 0.060 |
|
|
|
t-ZrO2: two formula units per cell and slight modification of fluorite structure. Zr (2a): (0 0 0) and O (4d): (0 0.5 Oz). |
|
|
Figure 2070c shows the variation of micro-hardness with deposition temperature for ZrO2.
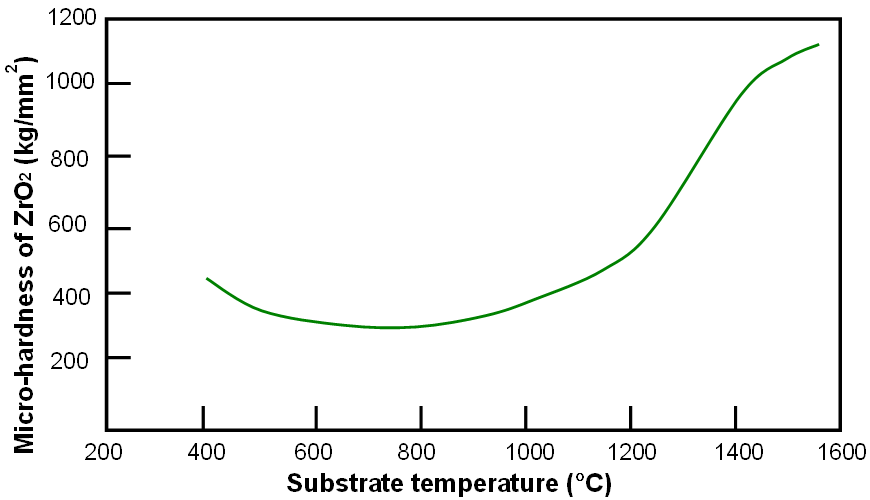
Figure 2070c. Variation of micro-hardness with deposition temperature for ZrO2.
ZrO2 has many applications because of:
i) Extremely high bending strength and tensile strength.
ii) High resistance to wear and to corrosion.
iii) High fracture toughness.
iv) Thermal expansion similar to cast iron.
v) Low thermal conductivity.
vi) Good oxygen ion conductivity.
vii) Very good tribological properties.
The applications of ZrO2 are mainly:
i) Piezoelectricity devices.
ii) PLZT ceramics.
iii) High-temperature passivation of microelectronic
devices.
iv) Structural composites.
v) Electrolytes.
vi) Oxygen sensors.
vii) Solid oxide fuel cells (SOFCs).
viii) Electronic
conduction coatings.
ix) Furnace elements.
x) Inorganic optical coatings.
In CVD process, unstabilized ZrO2 can be formed by the reaction of the metal halide with CO2 and hydrogen at 900-1200 °C:
ZrCl4 + 2CO2 + 2H2 → ZrO2 + CO + 4HCl
In the case of heated FEGs (field emission guns), also called Schottky emitters, the tip of the tungsten wire is coated with ZrO2 (zirconium oxide) to raise electrical conductivity.
[1] R. H. J. Hannink, P. M. Kelly, B. C. Muddle, 2000Transformation toughening in zirconia- containing ceramics. J Am Ceram Soc, 833Mar, 2000) 4614871551-2916
|

