=================================================================================
Table 4794b. Optimized options for elemental identification.
| 1 |
2 |
|
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
| 1 |
|
2 |
 XXXXXXXXXXXXXXXHXXXXXXXXXXXXXXXXX
XXXXXXXXXXXXXXXHXXXXXXXXXXXXXXXXX
EELS: Cannot be directly detected in general. However, it can be indirectly detected by detecting plasmon energy shift or chemical shift of other elements in some cases
EDS: Not possible
|
|
He |
| 3 |
4 |
|
5 |
6 |
7 |
8 |
9 |
10 |
| Li |
Be |
|
 XXXXXXXXXXXXXXXBXXXXXXXXXXXXXXXXX
XXXXXXXXXXXXXXXBXXXXXXXXXXXXXXXXX
1st choice: EELS energy window of 185.5~251 eV [Energy line (K 188 eV)]
2cd choice: EDS energy window of 0.173~0.193 keV [Energy line (Kα 0.183 keV)]
|
C |
N |
O |
F |
Ne |
| 11 |
12 |
|
13 |
14 |
15 |
16 |
17 |
18 |
| Na |
Mg |
|
Al |
Si |
P |
S |
Cl |
Ar |
| 19 |
20 |
|
21 |
22 |
23 |
24 |
25 |
26 |
27 |
28 |
29 |
30 |
31 |
32 |
33 |
34 |
35 |
36 |
| K |
Ca |
|
Sc |
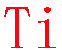 XXXXXXXXXXXXXXXTiXXXXXXXXXXXXXXXXX
XXXXXXXXXXXXXXXTiXXXXXXXXXXXXXXXXX
1st choice A: EDS energy window of 4.44~4.62 keV [Energy line (Kα 4.508 keV)]
1st choice B: EELS energy edge L3,2 455 eV
Last choice EDS: 0.41~0.48 keV Lα 0.452 keV
Intensity ratio: 1st choise A/last choice ratio = 4.6:1
|
V |
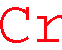 XXXXXXXXXXXXXXXCrXXXXXXXXXXXXXXXXX
XXXXXXXXXXXXXXXCrXXXXXXXXXXXXXXXXX
1st choice: EELS energy window of 574~645 eV [Energy line L3,2 575 eV)]
1st choice: EDS energy window of 5.32~5.53 keV [Energy lines (Kα 5.411 keV)]
2cd choice: EDS energy window of 5.86~6.05 KeV [Energy line Kβ1 5.946 KeV)]
3rd choice: EELS energy window of 695.5~709 eV [Energy lines (L1 695 eV)] (Very weak peak for verification)
|
Mn |
Fe |
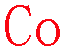 XXXXXXXXXXXXXXXCoXXXXXXXXXXXXXXXXX
XXXXXXXXXXXXXXXCoXXXXXXXXXXXXXXXXX
All below are good!
EELS: Energy window of 775~835 eV [Energy edge L3,2 779 eV]
EDS1: Energy window of 6.84~7.05 keV [Energy line (Kα 6.924 keV)]
EDS2: Energy window of 0.74~0.82 keV [Energy line (Lα 0.776 keV)]
EDS1:EDS2: Ratio: 1.4:1 |
 XXXXXXXXXXXXXXXNiXXXXXXXXXXXXXXXXX
XXXXXXXXXXXXXXXNiXXXXXXXXXXXXXXXXX
1st choice: EELS energy window of 846.5~1074 eV [Energy line (L3,2,1 855 eV)]
1st choice: EDS energy window of 7.38~7.59 keV [Energy line (Kα 7.461 keV)]
2cd choice: EDS energy window of 0.81~0.9 keV [Energy line (Lα,β 0.851 keV)]
Intensity ratio: 1st choice : 2cd choice = 1.7
Noisy choice for verification: EELS energy window of 59~73.5 eV [Energy line (M2,3 68 eV)]
No clear signal for samples thicker than 40 nm: EELS [Energy line (M1 112 eV)]
|
Cu |
Zn |
 XXXXXXXXXXXXXXXGaXXXXXXXXXXXXXXXXX
XXXXXXXXXXXXXXXGaXXXXXXXXXXXXXXXXX
1st choice: EDS energy window of 9.19~9.28 keV [Energy line (Kα 9.241 keV)]
3rd choice: EDS energy window of 10.2~10.38 keV [Energy line (Kβ 10.263 keV)]
Intensity ratio: 1st choice:3rd choice = 6.34:1
2nd choice: EDS energy window of 1.06~1.16 keV [Energy line Lα 1.098 keV]. Need deconvolution more often.
Intensity ratio: 2nd choice:1st choice = 2.25:1
|
Ge |
 XXXXXXXXXXXXXXXAsXXXXXXXXXXXXXXXXX
XXXXXXXXXXXXXXXAsXXXXXXXXXXXXXXXXX
1st choice: EDS energy window of 10.45-10.66 keV [Energy line (Kα 10.53 keV)]
2nd choice: EDS energy window of 1.25~1.35 window [Energy line (Lα 1.282 keV)]
Intensity ratio: 2nd choise/1st choice = 2.8:1
EELS L3,2 1.323 keV: Almost no signal for TEM samples with normal thicknesses
|
Se |
Br |
Kr |
| 37 |
38 |
|
39 |
40 |
41 |
42 |
43 |
44 |
45 |
46 |
47 |
48 |
49 |
50 |
51 |
52 |
53 |
54 |
| Rb |
Sr |
|
Y |
Zr |
Nb |
 XXXXXXXXXXXXXXXMoXXXXXXXXXXXXXXXXX
XXXXXXXXXXXXXXXMoXXXXXXXXXXXXXXXXX
1st choice A: For thick samples > EDS energy window 2.25~2.36 keV; [Energy line (Lα 2.293 keV)]; (Need deconvolution even with Si/Ga)
1st choice B: For thin samples: EELS energy window 222~272 eV [Energy edge M5,4 227 eV]
2nd choice: EDS energy window 17.33~17.64 keV; [Energy line (Kα 17.441 keV)]
Ratio of 1st Choice : 2nd Choice = 8.4:1 |
Tc |
 XXXXXXXXXXXXXXXRuXXXXXXXXXXXXXXXXX 1st choise: EDS energy window of 2.51~2.63 keV [Energy line (Lα 2.558 keV)]
XXXXXXXXXXXXXXXRuXXXXXXXXXXXXXXXXX 1st choise: EDS energy window of 2.51~2.63 keV [Energy line (Lα 2.558 keV)]
2nd choice: EDS energy window of 19.12~19.46 keV [Energy line (Kα 19.233 keV)]
Intensity ratio: 1st choise/2nd choice = 9.1 : 1
Good signal at EELS edge M5,4 279 eV, but it overlaps with C (Needs deconvolution if TEM is contaminated with carbon)
|
Rh |
Pd |
Ag |
Cd |
In |
Sn |
Sb |
Te |
I |
Xe |
| 55 |
56 |
|
71 |
72 |
73 |
74 |
75 |
76 |
77 |
78 |
79 |
80 |
81 |
82 |
83 |
84 |
85 |
86 |
| Cs |
Ba |
{57-70} |
Lu |
Hf |
Ta |
W |
Re |
Os |
Ir |
 XXXXXXXXXXXXXXXPtXXXXXXXXXXXXXXXXX
XXXXXXXXXXXXXXXPtXXXXXXXXXXXXXXXXX
1st choice: EDS energy window of 9.33~9.45 keV [Energy line Lα 9.441 keV]. Signal Overlap: Ga.
2nd choice: EDS energy window of 2.00-2.15 keV [Energy line M 2.048 keV]. Signal Overlap: P.
Intensity ratio: 1st choice/2nd choice = 1.77:1
Use one EDS energy window for confirmation: [10.92-11.16 keV], [11.16-11.36 keV], or [2.16-2.22 keV].
EELS: Not applicable in general
|
Au |
Hg |
Tl |
Pb |
Bi |
Po |
At |
Rn |
| 87 |
88 |
|
103 |
104 |
105 |
106 |
107 |
108 |
109 |
110 |
111 |
112 |
|
114 |
| Fr |
Ra |
[89-102] |
Lr |
Rf |
Db |
Sg |
Bh |
Hs |
Mt |
Ds |
Uuu |
Uub |
|
Uuq |
| |
|
57 |
58 |
59 |
60 |
61 |
62 |
63 |
64 |
65 |
66 |
67 |
68 |
69 |
70 |
|
|
| {lanthanides} {57-70} |
La |
Ce |
Pr |
Nd |
Pm |
Sm |
Eu |
Gd |
Tb |
Dy |
Ho |
Er |
Th |
Yb |
|
|
|
89 |
90 |
91 |
92 |
93 |
94 |
95 |
96 |
97 |
98 |
99 |
100 |
101 |
102 |
|
|
| {actinides} [89-102] |
Ac |
Th |
Pa |
U |
Np |
Pu |
Am |
Cm |
Bk |
Cf |
Es |
Fm |
Md |
No |
|
|
| |
The periodic table in Table 4794b lists the edge-onset energies in EEL spectra and energy peaks in EDS.
Table 4794b. Edge-onset energies in EEL spectra and energy peaks in EDS in the periodic table. The energy levels marked in red are practically detectable by both EDS and EELS methods.
| 1 |
2 |
|
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
| |
|
|
1 H
Hydrogen
EELS:
K 0.014 |
|
2 He
Helium
EELS:
K
0.025 |
| |
|
|
|
|
|
|
|
|
3 Li
Lithium
EELS:
K 0.055
|
4 Be
Beryllium
EDS:
Kα 0.110
EELS:
K 0.111
|
|
5 B
Boron
EDS:
Kα 0.183
EELS:
L2,3 0.005
K 0.188
|
6 C
Carbon
EDS:
Kα 0.277
EELS:
L2,3 0.007
K 0.284
|
7 N
Nitrogen
EDS:
Kα 0.392
EELS:
L2,3 0.009
K 0.399
|
8 O
Oxygen
EDS:
Kα 0.525
EELS:
L2,3 0.007
L1 0.024
K 0.532 |
9 F
Fluorine
EDS:
Kα 0.677
EELS:
L2,3 0.009
L1 0.031
K 0.686 |
10 Ne
Neon
EDS:
Kα 0.848
EELS:
L2,3 0.018
L1 0.045
K 0.867 |
| |
|
|
|
|
|
|
|
|
11 Na
Sodium
EDS:
Kα 1.041
EELS:
M1 1
L2,3 0.031 L1 0.063
K 1.072
|
12 Mg
Magnesium
EDS:
Kα 1.253
EELS:
M1 2
L2,3 0.052
L1 0.089
K 1.305
|
| |
Alkali earth |
|
|
Transition metals |
|
|
Halogens |
| |
Rare earth |
|
|
Noble gases |
|
|
Metalloids |
| |
Non-metals |
|
|
Alkaline earth |
|
|
Other metals |
|
13 Al
Aluminium
EDS:
Kα 1.486
EELS:
M1 0.001
L3 0.073
L2 0.074
L1 0.118
K 1.560
|
14 Si
Silicon
EDS:
Kα 1.739
EELS:
M2,3 0.003
M1 0.008
L3 0.099
L2 0.100
L1 0.149
K 1.839 |
15 P
Phosphorus
EDS:
Kα 2.013
EELS:
M2,3 0.010
M1 0.016
L3 0.135
L2 0.136
L1 0.189
|
16 S
Sulphur
EDS:
Kα 2.307
EELS:
M2,3 0.008
M1 0.016
L3 0.164
L2 0.165
L1 0.229
|
17 Cl
Chlorine
EDS:
Kα 2.621
EELS:
M2,3 0.007
M1 0.018
L3 0.200
L2 0.202
L1 0.270
|
18 Ar
Argon
EDS:
Kα 2.957
EELS:
M2,3 0.012
M1 0.025
L3 0.245
L2 0.247
L1 0.320
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
19 K
Potassium
EDS:
Kα 3.312
EELS:
M2,3 0.018
M1 0.034
L3 0.294
L2 0.297
L1 0.377
|
20 Ca
Calcium
EDS:
Kα 3.690
Lα
0.341
EELS:
M4,5 0.005
M2,3 0.026
M1 0.044
L3 0.347
L2 0.350
L1 0.438
|
|
21 Sc
Scandium
EDS:
Kα 4.088
Lα 0.395
EELS:
M4,5 0.007
M2,3 0.032
M1 0.054
L3 0.402
L2 0.407
L1 0.500
|
22 Ti
Titanium
EDS:
Kα 4.508
Lα 0.452
EELS:
M4,5 0.003
M2,3 0.034
M1 0.059
L3 0.455
L2 0.461
L1 0.564
|
23 V
Vanadium
EDS:
Kα 4.949
Lα 0.511
EELS:
M4,5 0.002
M2,3 0.038
M1 0.066
L3 0.513
L2 0.520
L1 0.628
|
24 Cr
Chromium
EDS:
Kα 5.411
Lα 0.573
EELS:
M4,5 0.002
M2,3 0.043
M1 0.074
L3 0.575
L2 0.584
L1 0.695
|
25 Mn
Manganese
EDS:
Kα 5.894
Lα 0.637
EELS:
M4,5 0.004
M2,3 0.049
M1 0.084
L3 0.641
L2 0.652
L1 0.769
|
26 Fe
Iron
EDS:
Kα 6.398
Lα 0.705
EELS:
M4,5 0.006
M2,3 0.056
M1 0.095
L3 0.710
L2 0.723
L1 0.846
|
27 Co
Cobalt
EDS:
Kα 6.924
Lα 0.776
EELS:
M4,5 0.003
M2,3 0.060
M1 0.101
L3 0.779
L2 0.794
L1 0.926
|
28 Ni
Nickel
EDS:
Kα 7.471
Lα 0.851
EELS:
M4,5 0.004
M2,3 0.068
M1 0.112
L3 0.855
L2 0.872
L1 1.008
|
29 Cu
Copper
EDS:
Kα 8.040
Lα 0.930
EELS:
M4,5 0.002
M2,3 0.074
M1 0.120
L3 0.931
L2 0.951
L1 1.096
|
30 Zn
Zinc
EDS:
Kα 8.630
Lα 1.012
EELS:
M4,5 0.009
M2,3 0.087
M1 0.137
L3 1.021
L2 1.044
L1 1.194
|
31 Ga
Gallium
EDS:
Kα 9.241
Lα 1.098
EELS:
N2,3 0.001
M4,5 0.018
M3 0.103
M2 0.107
L3 1.116
L2 1.143
L1 1.298
|
32 Ge
Germanium
EDS:
Kα 9.874
Lα 1.188
EELS:
N2,3 0.003
M4,5 0.029
M3 0.122
M2 0.129
L3 1.217
L2 1.249
L1 1.414
|
33 As
Arsenic
EDS:
Kα 10.530
Lα 1.282
EELS:
N2,3 0.003
M4,5 0.041
M3 0.141
M2 0.147
L3 1.323
L2 1.359
L1 1.527
|
34 Se
Selenium
EDS:
Kα 11.207
Lα 1.379
EELS:
N2,3 0.006
M4,5 0.057
M3 0.162
M2 0.168
L3 1.436
L2 1.476
L1 1.654
|
35 Br
Bromine
EDS:
Kα 11.907
Lα 1.480
EELS:
N2,3 0.005
N1 0.027
M5 0.069
M4 0.070
M3 0.182
L3 1.551
L2 1.597
L1 1.783
|
36 Kr
Krypton
EDS:
Kα 12.631
Lα 1.586
EELS:
N2,3 0.011
N1 0.024
M4,5 0.089
M3 0.214
M2 0.223
L3 1.675
L2 1.727
L1 1.921
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
37 Rb
Rubidium
EDS:
Kα 13.373
Lα 1.694
EELS:
N2,3 0.014
N1 0.030
M5 0.111
M4 0.112
M3 0.239
L3 1.806
L2 1.865
|
38 Sr
Strontium
EDS:
Kα 14.140
Lα 1.806
EELS:
N2,3 0.020
N1 0.038
M5 0.133
M4 0.135
M3 0.269
L3 1.941
|
|
39 Y
Yttrium
EDS:
Kα 14.931
Lα 1.922
EELS:
N4,5 0.003
N2,3 0.026
N1 0.046
M5 0.158
M4 0.160
M3 0.301
|
40 Zr
Zirconium
EDS:
Kα 15.744
Lα 2.042
EELS:
N4,5 0.003
N2,3 0.029
N1 0.052
M5 0.180
M4 0.183
M3 0.331
|
41 Nb
Niobium
EDS:
Kα 16.581
Lα 2.166
EELS:
N4,5 0.004
N2,3 0.034
N1 0.058
M5 0.205
M4 0.208
M3 0.363
|
42 Mo
Molybdenum
EDS:
Kα 17.441
Lα 2.293
EELS:
N4,5 0.002
N2,3 0.035
N1 0.062
M5 0.227
M4 0.230
M3 0.393
|
43 Tc
Technetium
EDS:
Kα 18.325
Lα 2.424
EELS:
N4,5 0.002
N2,3 0.039
N1 0.068
M5 0.253
M4 0.257
M3 0.425
|
44 Ru
Ruthenium
EDS:
Kα 19.233
Lα 2.558
EELS:
N4,5 0.002
N2,3 0.043
N1 0.075
M5 0.279
M4 0.284
M3 0.461
|
45 Rh
Rhodium
EDS:
Lα 2.696
EELS:
N4,5 0.003
N2,3 0.048
N1 0.081
M5 0.307
M4 0.312
M3 0.496
|
46 Pd
Palladium
EDS:
Lα 2.838
EELS:
N4,5 0.001
N2,3 0.051
N1 0.086
M5 0.335
M4 0.340
M3 0.531
|
47 Ag
Silver
EDS:
Lα 2.984
EELS:
N4,5 0.003
N3 0.056
N2 0.062
M5 0.367
M4 0.373
M3 0.571
|
48 Cd
Cadmium
EDS:
Lα 3.133
EELS:
O2,3 0.002
N4,5 0.009
N2,3 0.067
N1 0.108
M5 0.404
M4 0.411
M3 0.617
|
49 In
Indium
EDS:
Lα 3.286
M 0.368
EELS:
O2,3 0.001
N4,5 0.016
N2,3 0.077
N1 0.122
M5 0.443
M4 0.451
M3 0.664
|
50 Sn
Tin
EDS:
Lα 3.443
M 0.691
EELS:
O2,3 0.001
N4,5 0.024
N2,3 0.089
N1 0.137
M5 0.485
M4 0.494
M3 0.715
|
51 Sb
Antimony
EDS:
Lα 3.604
M 0.733
EELS:
O2,3 0.002
O1 0.007
N4,5 0.032
N2,3 0.099
N1 0.152
M5 0.528
M4 0.537
M3 0.766 |
52 Te
Tellurium
EDS:
Lα 3.769
M 0.778
EELS:
O2,3 0.002
O1 0.012
N4,5 0.040
N2,3 0.110
N1 0.168
M5 0.572
M4 0.582
M3 0.819 |
53 I
Iodine
EDS:
Lα 3.937
Kα1 28.615
EELS:
O2,3 0.003
O1 0.014
N4,5 0.050
N2,3 0.123
N1 0.186
M5 0.620
M4 0.631
M3 0.875 |
54 Xe
Xenon
EDS:
Lα 4.109
Kα1 29.779
EELS:
O2,3 0.007
O1 0.018
N4,5 0.063
N2,3 0.147
N1 0.208
M5 0.672
M4 0.685
M3 0.937 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
55 Cs
Cesium
EDS:
Kα 30.971
Lα 4.286
EELS:
O2,3 0.012
O1 0.023
N5 0.077
N4 0.079
N3 0.162
M5 0.726
M4 0.740
M3 0.998
|
56 Ba
Barium
EDS:
Kα 32.196
Lα 4.465
M 0.972
EELS:
O3 0.015
O2 0.017
O1 0.040
N5 0.090
N4 0.093
N3 0.180
M5 0.781
M4 0.796
M3 1.063 |
{57-70}
Lanthanoid |
71 Lu
Lutetium
EDS:
Lα 7.654
M 1.581
EELS:
O4,5 0.005
O2,3 0.028
O1 0.057
N6,7 0.007
N5 0.195
N4 0.205
M5 1.588
M4 1.639
|
72 Hf
Hafnium
EDS:
Lα 7.898
M 1.644
EELS:
O4,5 0.007
O3 0.031
O2 0.038
N7 0.018
N6 0.019
N5 0.214
M5 1.662
M4 1.716
|
73 Ta
Tantalum
EDS:
Lα 8.145
M 1.709
EELS:
O4,5 0.006
O3 0.037
O2 0.045
N7 0.025
N6 0.027
N5 0.230
M5 1.735
M4 1.793
|
74 W
Tungsten
EDS:
Lα 8.396
M 1.774
EELS:
O4,5 0.006
O3 0.037
O2 0.047
N7 0.034
N6 0.037
N5 0.246
M5 1.810
M4 1.872
|
75 Re
Rhenium
EDS:
Lα 8.651
M 1.842
EELS:
O4,5 0.004
O3 0.035
O2 0.046
N7 0.045
N6 0.047
N5 0.260
M5 1.883
M4 1.949
|
76 Os
Osmium
EDS:
Lα 8.910
M 1.914
EELS:
O3 0.046
O2 0.058
N7 0.050
N6 0.052
N5 0.273
M5 1.960
|
77 Ir
Iridium
EDS:
Lα 9.174
M 1.977
EELS:
O4,5 0.004
O3 0.051
O2 0.063
N7 0.060
N6 0.063
N5 0.295
|
78 Pt
Platinum
EDS:
Lα 9.441
M 2.048
EELS:
O4,5 0.002
O3 0.051
O2 0.066
N7 0.070
N6 0.074
N5 0.314
|
79 Au
Gold
EDS:
Lα 9.712
M 2.120
EELS:
O4,5 0.003
O3 0.054
O2 0.072
N7 0.083
N6 0.087
N5 0.334
|
80 Hg
Mercury
EDS:
Lα 9.987
M 2.195
EELS:
O4,5 0.007
O3 0.058
O2 0.081
N7 0.099
N6 0.103
N5 0.360
|
81 Tl
Thallium
EDS:
Lα 10.267
M 2.267
EELS:
O5 0.013
O4 0.016
O3 0.076
N7 0.118
N6 0.122
N5 0.386
|
82 Pb
Lead
EDS:
Lα 10.550
M 2.342
EELS:
P2,3 0.001
P1 0.003
O5 0.020
O4 0.022
O3 0.086
N7 0.138
N6 0.143
N5 0.413
|
83 Bi
Bismuth
EDS:
Lα 10.837
M 2.419
EELS:
P2,3 0.003
P1 0.008
O5 0.025
O4 0.027
O3 0.093
N7 0.158
N6 0.163
N5 0.440
|
84 Po
Polonium
EDS:
Lα 11.129
EELS:
P2,3 0.005
P1 0.012
O4,5 0.031
O3 0.104
O2 0.132
N6,7 0.184
N5 0.473
N4 0.500
|
85 At
Astatine
EDS:
Lα 11.425
EELS:
P2,3 0.008
P1 0.018
O4,5 0.040
O3 0.115
O2 0.148
N6,7 0.210
N5 0.507
N4 0.533
|
86 Rn
Radon
EDS:
Lα 11.725
EELS:
P2,3 0.011
P1 0.026
O4,5 0.048
O3 0.127
O2 0.164
N6,7 0.238
N5 0.541
N4 0.567
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
87 Fr
Francium
EDS:
Lα 12.029
EELS:
P2,3 0.015
P1 0.034
O4,5 0.058
O3 0.140
O2 0.182
N6,7 0.268
N5 0.577
N4 0.603 |
88 Ra
Radium
EDS:
Lα 12.340
EELS:
P2,3 0.019
P1 0.044
O4,5 0.068
O3 0.153
O2 0.200
N6,7 0.299
N5 0.603
N4 0.636 |
[89-102]
Actinoid |
103 Lr
Lawrencium
|
104 Rf
Rutherfordium
|
105 Db
Dubnium
|
106 Sg
Seaborgium
|
107 Bh
Bohrium
|
108 Hs
Hassium
|
109 Mt
Meitnerium
|
110 Uun
Ununnilium
|
111 Uuu
Unununium
|
112 Uub
Ununbium
|
|
114 Uuq
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| {lanthanides} {57-70} |
57 La
Lanthanum
EDS:
Kα 33.441
Lα 4.650
M 0.833
EELS:
O2,3 0.015
O1 0.033
N4,5 0.099
N3 0.192
N2 0.206
M5 0.832
M4 0.849
M3 1.124 |
58 Ce
Cerium
EDS:
Kα 34.717
Lα 4.839
M 0.883
EELS:
O2,3 0.020
O1 0.038
N6,7 0.001
N4,5 0.111
N3 0.208
M5 0.884
M4 0.902
M3 1.186 |
59 Pr
Praseodymium
EDS:
Kα 36.031
Lα 5.033
M 0.929
EELS:
O2,3 0.023
O1 0.038
N6,7 0.002
N4,5 0.114
N3 0.218
M5 0.931
M4 0.951
M3 1.243 |
60 Nd
Neodymium
EDS:
Kα 37.358
Lα 5.229
M 0.978
EELS:
O2,3 0.022
O1 0.038
N6,7 0.002
N4,5 0.118
N3 0.225
M5 0.978
M4 1.000
M3 1.298 |
61 Pm
Prometium
EDS:
Kα 38.725
Lα 5.432
EELS:
O2,3 0.022
O1 0.038
N6,7 0.004
N4,5 0.121
N3 0.237
M5 1.027
M4 1.052
M3 1.357 |
62 Sm
Samarium
EDS:
Lα 5.635
M 1.081
EELS:
O2,3 0.022
O1 0.039
N6,7 0.007
N4,5 0.130
N3 0.249
M5 1.081
M4 1.107
M3 1.421 |
63 Eu
Europium
EDS:
Lα 5.845
M 1.131
EELS:
O2,3 0.022
O1 0.032
N4,5 0.134
N3 0.257
M5 1.131
M4 1.161
M3 1.481
|
64 Gd
Gadolinium
EDS:
Lα 6.056
M 1.185
EELS:
O2,3 0.021
O1 0.036
N4,5 0.141
N3 0.271
M5 1.186
M4 1.218
M3 1.544
|
65 Tb
Terbium
EDS:
Lα 6.272
M 1.240
EELS:
O2,3 0.026
O1 0.040
N6,7 0.003
N4,5 0.148
N3 0.286
M5 1.242
M4 1.276
M3 1.612 |
66 Dy
Dysprosium
EDS:
Lα 6.494
M 1.293
EELS:
O2,3 0.026
O1 0.063
N6,7 0.004
N4,5 0.154
N3 0.293
M5 1.295
M4 1.332
M3 1.676 |
67 Ho
Holmium
EDS:
Lα 6.719
M 1.347
EELS:
O2,3 0.020
O1 0.051
N6,7 0.004
N4,5 0.161
N3 0.306
M5 1.351
M4 1.391
M3 1.741
|
68 Er
Erbium
EDS:
Lα 6.947
M 1.405
EELS:
O2,3 0.029
O1 0.060
N6,7 0.004
N5 0.168
N4 0.177
M5 1.409
M4 1.453
M3 1.812 |
69 Th
Thulium
EDS:
Lα 7.179
M 1.462
EELS:
O2,3 0.032
O1 0.053
N6,7 0.005
N4,5 0.180
N3 0.337
M5 1.468
M4 1.515
M3 1.885 |
70 Yb
Ytterbium
EDS:
Lα 7.414
M 1.521
EELS:
O2,3 0.023
O1 0.053
N6,7 0.006
N5 0.184
N4 0.197
M5 1.527
M4 1.576
M3 1.949 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| {actinides} [89-102] |
89 Ac
Actinium
EDS:
Lα 12.650
EELS:
O4,5 0.080
O3 0.167
O2 0.215
N6,7 0.319
N5 0.639
N4 0.675
|
90 Th
Thorium
EDS:
Lα 12.967
M 2.991
EELS:
P4,5 0.002
P3 0.043
P2 0.049
O5 0.088
O4 0.095
O3 0.182
N7 0.335
N6 0.344
N5 0.677 |
91 Pa
Protactinium
EDS:
Lα 13.288
M 3.077
EELS:
O5,6 0.094
O2,3 0.223
O1 0.310
N7 0.360
N6 0.371
N5 0.708
|
92 U
Uranium
EDS:
Lα 13.612
M 3.164
EELS:
P4,5 0.004
P3 0.033
P2 0.043
O5 0.096
O4 0.105
O3 0.195
N7 0.381
N6 0.392
N5 0.738 |
93 Np
Neptunium
EDS:
Lα 13.942
M 3.260
EELS:
O5 0.101
O4 0.109
O3 0.206
N7 0.404
N6 0.415
N5 0.773
|
94 Pu
Plutonium
EDS:
Lα 14.276
M 3.348
EELS:
O5 0.105
O4 0.116
O3 0.212
N6,7 0.422
N5 0.801
N4 0.849
|
95 Am
Americium
EDS:
Lα 14.615
M 3.437
EELS:
O5 0.103
O4 0.116
O3 0.220
N6,7 0.440
N5 0.828
N4 0.879
|
96 Cm
Curium
EDS:
Lα 14.953
M 3.539
|
97 Bk
Berkelium
EDS:
Lα 15.304
M 3.634
|
98 Cf
Californium
EDS:
Lα 15.652
M 3.731
|
99 Es
Einsteinium
|
100 Fm
Fermium
|
101 Md
Mendelevium
|
102 No
Nobelium
|
|
|
| |
Table 4794c and 4794d list the comparison between EDS and EELS methods.
| Table 4794c. Comparison table between EDS and EELS methods for operators and microscopists. |
|
EDS |
EELS |
Naming |
Named by the initial core-hole state, the emission line, and the line strength (1 is the strongest), e.g. 3d5/2 → 2p3/2 is Lα1 [see page4478] |
Named by the initial state, , e.g. 2p3/2 → 3d5/2 is Lα1 [see page4478] |
| |
Parameters to maximize counts and minimize analysis time |
Accelerating voltage |
Lower (120 kV or 80 kV) |
Concentration |
Larger, but fixed by the specimen |
Map time |
Longer, but limited by throughput and specimen damage |
Pixel time |
Longer, but limited by specimen damage, throughput, and drift correction |
Probe correction |
More current in primary beam and less in tails |
Probe current |
Larger (100 pA to 5 nA), but limited by specimen damage and element diffusion |
Detector area
|
Larger, but there are more spurious peaks and shadowing |
Larger, but limited by STEM detector |
Sample mounting |
Prevent shadowing from TEM grid |
No limitation |
|
Others |
Signal to background ratio |
E.g. F K peak: 4 ± 1; Na K peak: 16 ± 4; Na L3,2 peak: 16 ± 4; P L3,2 peak: 3 ± 1; Cl L3,2 peak: 20 ± 5 [2]
|
E.g. F K edge: 0.39 ± 0.06; Na K edge: 0.23 ± 0.04; Na L3,2 edge: 0.17 ± 0.03; P L3,2 edge: 0.016 ± 0.003; Cl L3,2 edge: 0.10 ± 0.02 [2], and C K 5.2 for 100 mrad, 16 for 50 mrad and 37 for 5 mrad [3]. |
Signal to noise ratio |
High: is largely limited by counting statistics (noise is approximately equal to the square root of the signal for thin TEM samples) |
Low: is limited by the total signal, including the edge and the underlying background |
Signal |
Lower signal for elements with smaller atomic number |
Higher core-loss signal in general |
Acquisition time |
Typically is one to two orders of magnitude longer than for EELS |
Shorter for lighter elements, while becoming equal to EDS for heavier elements |
Acquisition speed |
Fast: The total spectrum of interest, from 0.1 keV to the beam energy (e.g., 20 keV) can be acquired in a short time (10 - 100 s). |
Slow |
Efficiency to atomic number |
Less efficient for lighter atoms (say Z < 30), especially not lighter than B. |
Better range, but more efficient for lighter atoms |
|
Smaller ionization cross sections |
Larger ionization cross sections |
Collection efficiency |
|
|
Background |
Lower spectral background |
Higher spectral background: rapid variation of the background |
Process & transition |
X-ray generation can originate from energy loss process of incident electrons. Refer to Figures 4794b and 4794c below. |
Energy loss process is the first step after interaction of incident electrons with atoms. Refer to Figures 4794b and 4794c below. |
Energy position |
Lower. E.g. Yb M-lines (Mα): 1.52 keV; Al K-lines (Kα): 1.49 keV; Si K-lines (Kα): 1.74 keV |
Higher. E.g. Yb-M4,5: 1.528 keV; Al-K: 1.56 keV, Si-K: 1.839 keV |
Energy resolution |
The typical energy resolution
for EDS is of on the order of 120 ~ 150 eV. This is suffcient in most cases
for resolving peaks of different elements, but is inadequate for detecting chemical shifts of the atoms which are of the order of a few electronvolts. EDS
is thus mainly used for composition analysis. |
< 1 eV. The better resolution provides more information: enables analysis of electronic structure (density of empty states, oxidation state, local coordination, bandgap, ...)
|
Resolution affected by beam broadening in TEM specimen |
Strongly (refer to Figure 4794a below) |
Weakly (also refer to Figure 4794a below) |
Quantification |
Standard needed: The relative intensities of the peaks depend on the properties of the detector; Applicable for most cases. |
Standardless and absolute quantification: The intensity of EELS signal mostly depends on the TEM specimen but does not depend on the properties of the detector (spectrometer) and the structure of the microscope; therefore, the elemental quantification based on EELS does not require specimen standards. Possible errors. |
Artefacts |
|
No stray etc. |
| Features |
Always immediately visible |
Not always immediately visible |
Signal delocalization by X-ray fluorescence |
Is a problem |
Is not a problem |
Operator experience |
Less operator intensive; easy and quick; easy data interpretation |
More operator intensive; need skill; difficult data interpretation |
Disadvantage |
Easier interpretation |
Interpretation can be difficult |
| Table 4794d. Comparison table between EDS and EELS methods for the people who use EDS and EELS data. |
|
EDS |
EELS |
| |
Sample thickness |
Thicker (20 - 500 nm) |
Thinner (10–50 nm) |
Optimized thickness for TEM |
50-100 nm thick |
Less than 20-50 nm thick,depending on materials (e.g. 37 nm for C, 33 nm for Cu, 36 nm for HfOx, 48 nm for Si, 52 nm for SiO2, 36 nm for WO3, and 46 nm for TiN) |
Elements preferred |
Heavy elements (Higher Z-numbe) |
Light elements (Lower Z-numbe) |
Sensitivity |
|
|
Spatial resolution |
Beam broadening, so lower: > 2 nm |
Higher ultimate spatial resolution (~ 1 nm) because the angular distribution of inelastic scattering is strongly forward peaked. |
Mismatch of quantification |
E.g. Al : Si : Yb compositions of at% 43 : 47 : 10 in EELS and at% 48 : 35 : 16 for EDS for a same material [1] |
Data accuracy depending on grain orientation |
Less dependence on crystal orientation: Better (< 5%) |
Large inaccuracy (±10%) in crystals due to orientation dependence at long camera lengths; small inaccuracy (±1%) in crystals due to orientation dependence at short camera lengths |
Structural information (e.g. bonding and structure information) |
Not sensitive |
Available |
Signal (contrast) reversal |
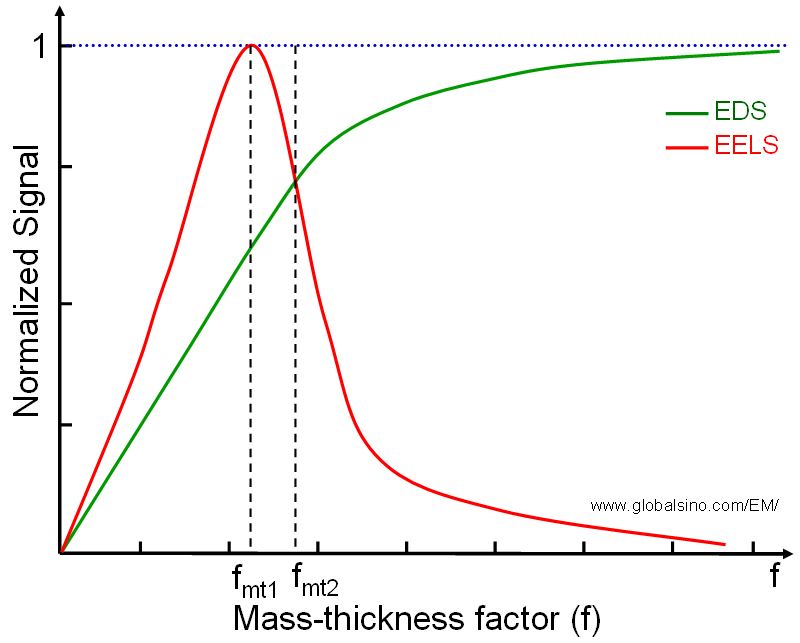
| The EELS signal becomes smaller than EDS signal when f is greater than fms2. Therefore, the EDS and EELS signals (contrasts) will be reversed between the cases of f < fms1 and f >fms2 (see page1385 and page1386 for details). That is, in the case of f >fms2, higher concentration of an specific element probably does not provide high intensity or contrast in EELS measurements, but it does for EDS. |
|
|
Dependence on EM sample thickness |
Signal intensity is a problem from a thin specimen |
Signal intensity is better for thin specimen; very thin specimen is needed |
Applicability |
Can be
conducted on both thick and thin specimens and can be conducted in both
SEM and TEM, so that it is most frequently purchased
electron microscope accessory for elemental analysis |
Requires very
thin specimens and the analysis is more complicated, so that it is less common. However, the information can
be highly valuable |
Figure 4794a shows the schematic illustration of the broadening of electron beam within a thin specimen and of generations of EDS, EELS and AES signals. This beam broadening affects the spatial resolution of EDS significantly, but does not affect those of EELS and AES too much. d, R and Rmax are the spot size , the average diameter, and the maximum diameter of the electron beam within the specimen, respecitively. α and β are convergence semiangle of the electron beam and the collection semiangle of EELS/EFTEM. The angle-limiting aperture can be the objective aperture of TEM/STEM system or the entrance aperture of the EELS system, which is smaller and dominates the beam intensity arriving at the camera.
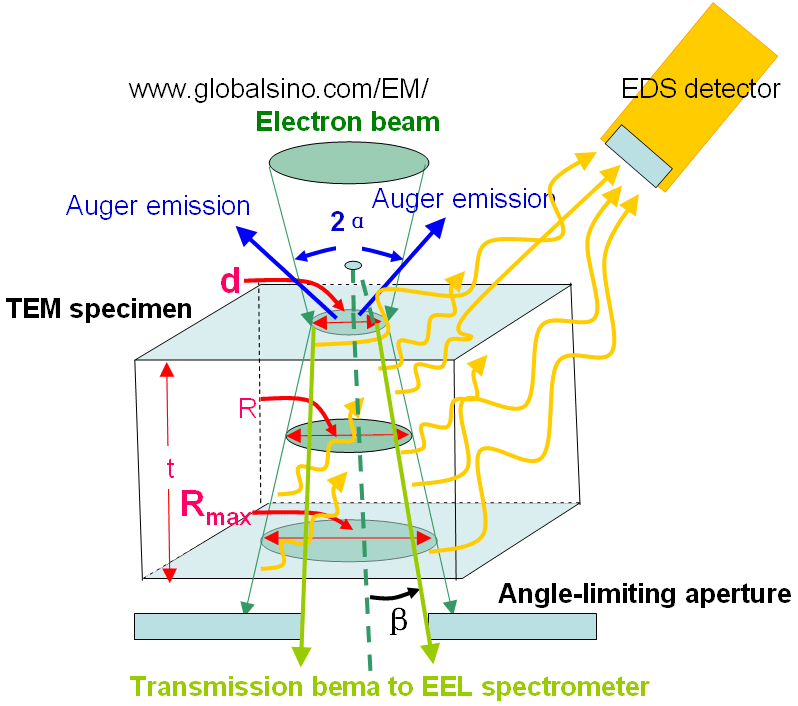
Figure 4794a. Schematic illustration of the broadening of electron beam
within a thin specimen and of generations of EDS, EELS and AES signals.
Figure 4794b shows the schematic illustrations of examples of energy loss process of incident electrons (a) and x-ray generation (b). EKE1 and EKE2 represent the kinetic energies of the two generated SEs. ΔE1 and ΔE2 represent the energy losses of the incident electrons after the incident electrons interact with the electrons in the K and L3 subshells, respectively. E1 and E2 are the binding energies of the two electrons. E0 is the energy of the incident electrons in the EMs.
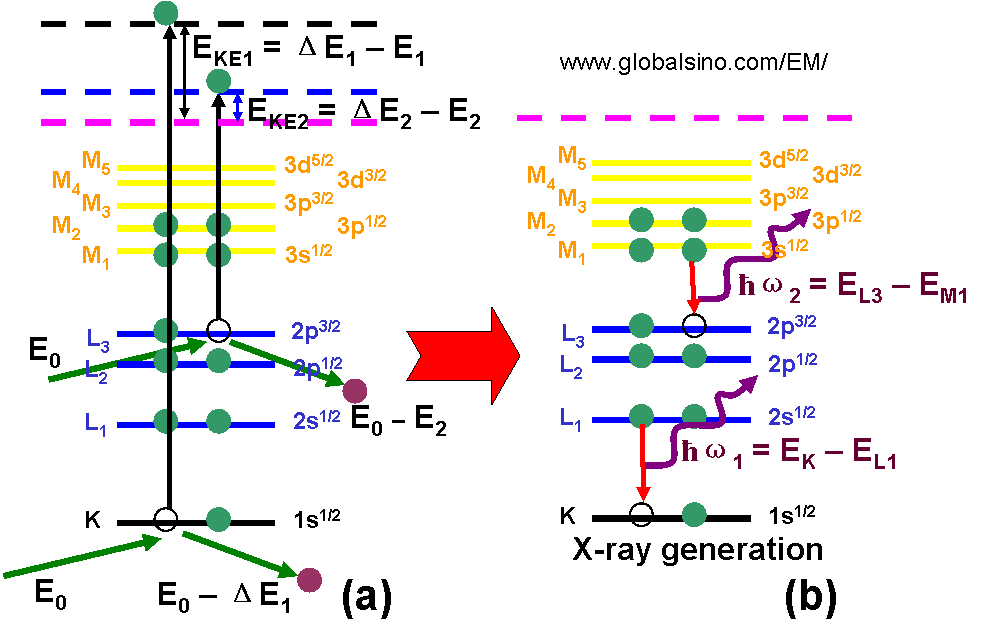
Figure 4794b. Schematic illustrations of examples of energy loss process (a) and x-ray generation (b).
Figure 4794c lists the EELS and EDS transitions. Note that the EELS transitions are named by the initial state, while EDS transitions are named by the initial core-hole state, the emission line, and the line strength (1 is the strongest).
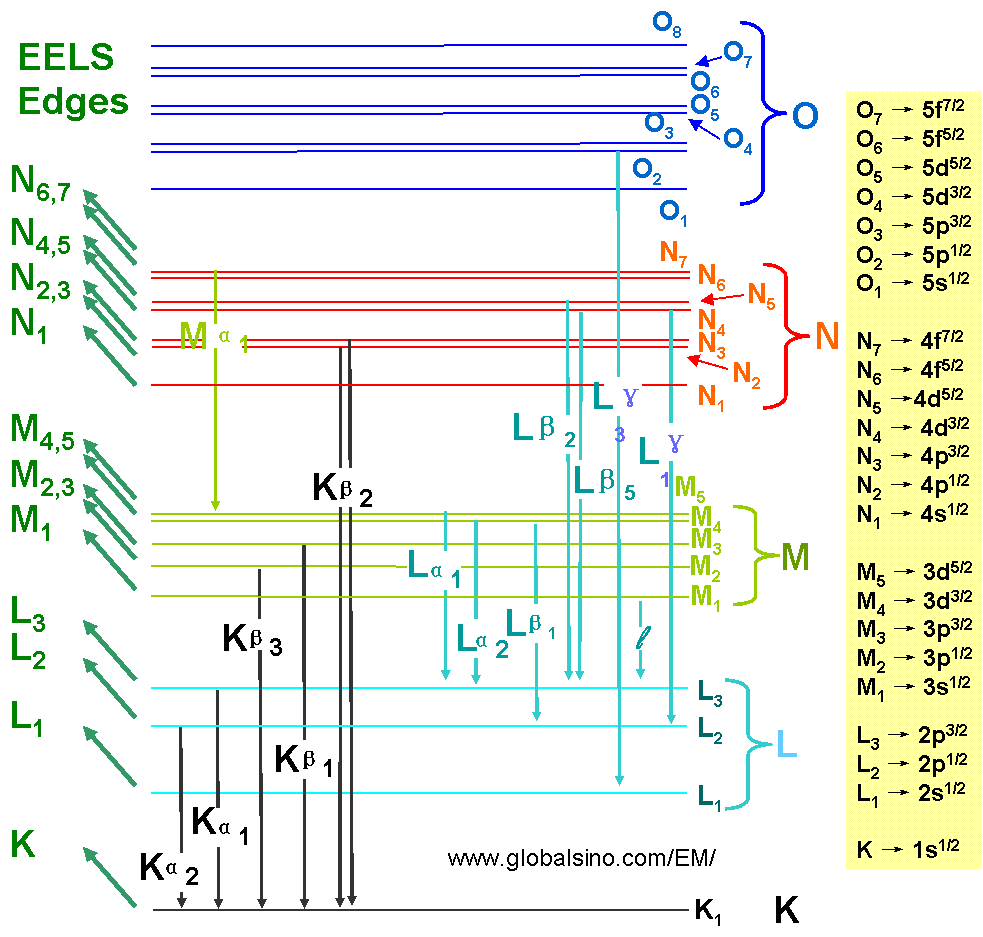
Figure 4794c. EELS and EDS transitions.
[1] Georg Haberfehlner, Angelina Orthacker, Mihaela Albu, Jiehua Li and Gerald Kothleitner, Nanoscale voxel spectroscopy by simultaneous EELS and EDS tomography, Nanoscale, 2014, 6, 14563.
[2] Richard D. Leapman and John A. Hunt, Comparison of detection limits for EELS and EDXS, Microsc. Microanal. Micronstruct., 2 (1991) 231-244.
[3] Egerton, R.F., Inelastic scattering of 80 keV electrons in amorphous carbon, Phil. Mag. 31, pp. 199-215 (1975).
|